
Jasmine Grover Content Strategy Manager
Content Strategy Manager
Thermochemistry deals with the transmission of heat between the system and its surroundings. It is the study of the energy changes involved in various physical and chemical changes and reactions. In this article, we will deal with thermochemistry reactions, system and surroundings, sign conventions, and calorimetry.
Read More: NCERT Solutions for Class 11 Thermodynamics
| Table of Contents |
Key Terms:- Thermochemistry, chemical reaction, Reactants, absorption and liberation of energy, calorimetry, Energy change.
What is Thermochemistry?
[Click Here for Sample Questions]
During the course of a chemical reaction, reactants combine together to form products. This process involves the breaking and formation of bonds. Since the breaking and formation of bonds involve absorption and liberation of energy respectively, chemical reactions are always accompanied by energy changes.
Sometimes, the energy change associated with the chemical reaction is more significant than the reaction itself. Therefore, the study of the energy changes involved in various physical and chemical changes is of great importance. The branch of science which deals with the energy changes associated with these chemical reactions is called Thermochemistry.
Thermochemistry
Read More:
| Concepts Related to Thermochemistry | ||
|---|---|---|
| Carnot engine | Zeroth law of Thermodynamics | Second law of thermodynamics |
| Isobaric process | Efficiency Formula | first law of thermodynamics |
System and Surrounding
[Click Here for Previous years Questions]
System: A system refers to any specified portion of matter under consideration which is kept apart from the rest of the universe with a bounding surface.
Surroundings: Surroundings refers to the remainder of the universe that could be in a position to interchange energy and matter with the system.
| System + Surroundings = Universe |
For example, When a given amount of gas is enclosed in a container, the gas present within the walls of the container acts as a system where the walls of the container are the boundaries. Everything outside the container is called surroundings.

System and Surrounding
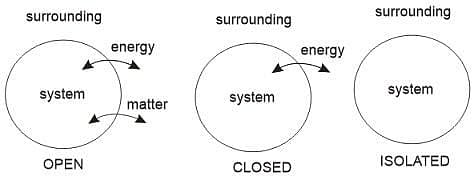
Types Of System
[Click Here for Sample Questions]
The system is generally classified into 3 types:
- Isolated system: An isolated system is one that is unable to exchange energy or matter with its surroundings.
Example: Thermos Flask.
- Closed system: A closed system is one that can interchange energy but not matter with its surroundings.
Example: A cup of tea covered with a lid.
- Open system: An open system is a system that can exchange both matter as well as energy with its surroundings.
Example: A beaker filled with water.
Types Of System
Thermochemistry Terminology:
- Extensive properties: An extensive property of a system is that which depends upon the amount of the substance present in the system like mass, volume, and energy.
- Intensive properties: An intensive property of a system is that which is independent of the amount of the substance present. Intensive properties can be like temperature, specific heat, density, pressure, viscosity, refractive index, and surface tension.
- State Function and State Variable: The fundamental properties pressure, volume, temperature, internal energy, enthalpy, entropy, etc. which define the state of a system are known as state functions or state variables.
Thermochemical Reaction
[Click Here for Previous years Questions]
A thermochemical reaction refers to an equation that represents the physical state of all the products and reactants with a balanced chemical reaction. It also accounts for the change in heat.
Types of Thermochemical Reaction:-
- Endothermic Reaction: Any process that causes the system's enthalpy H (or internal energy U) to rise is called an endothermic process. A closed system absorbs thermal energy from its surroundings throughout this phase, resulting in heat transfer into the system. It could be a chemical or physical process, such as dissolving ammonium nitrate in water.
- Exothermic Reaction: Any process that causes the system's enthalpy H (or internal energy U) to fall is called an exothermic process. A closed system releases thermal energy to its surroundings throughout this phase, resulting in heat transfer into the surroundings. It could be a chemical or physical process, such as combustion.
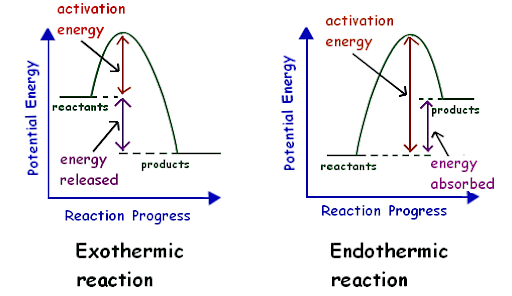
Thermochemical reaction
Sign Conventions in Thermochemistry
- If the heat is absorbed by the system (q>0) then the reaction is said to be endothermic and ΔE or ΔH value is given a positive sign.
- If the heat is evolved (q<0) the reaction is said to be exothermic, and ΔE or ΔH value is given a negative sign.
Standard States
[Click Here for Sample Questions]
It is common practice to assume that the heat of production of elements in their standard states is zero when computing the heat of reactions. The standard state is defined as the state at a constant temperature and pressure of 1 atm. The following are the standard states for several types of matter:
| State | Standard State |
|---|---|
| Gas | At 1 atm pressure and the constant given temperature, it acts as an ideal gas. |
| Liquid | At 1 atm pressure and the constant given temperature, it acts as pure liquid. |
| Solid | Stable crystalline form at 1atm and given temperature (example, graphite form of carbon, a rhombic form of sulfur). |
The heat of reactions is indicated by E° or H° at a certain temperature in the standard state.
Types of Enthalpies of Reaction
[Click Here for Previous years Questions]
- Enthalpy of Formation: Enthalpy changes when one mole of a given compound is formed from its elements.
H2(g) + 1/2O2(g) → 2H2O(1) ΔH = -890.36 kJ/mol
- Enthalpy of Combustion: Enthalpy changes when one mole of a substance is burnt in oxygen.
CH4 + 2O2(g) → CO2 + 2H2O(1) ΔH = -890.36 kJ/mol
- Enthalpy of Neutralization: The changes taking place in the enthalpy when an acid is neutralized by a base in a dilute solution. Since they entirely dissolve into ions in dilute solutions, this is a constant with a value of -13.7 kcal for neutralization of any strong acid or strong base.
H+ (aq) + OH- (aq) → H2O(l) ΔH = -13.7 kcal
Because weak acids and bases are not entirely dissociated, some heat is absorbed during dissociation, the heat of neutralization is not constant. Thus, the total heat released during neutralization will be less.
HCN + NaOH → NaCN + H2O ΔH = -2.9 kcal
In this process, the heat of ionization is equivalent to (-2.9 + 13.7) kcal = 10.8 kcal.
- Enthalpy of Hydration: Enthalpy of hydration of a given anhydrous or partially hydrated salt refers to the amount of change in enthalpy when it combines with the requisite number of moles of water in order to form a particular hydrate.
The hydration of anhydrous copper sulphate, for example, is given by
CuSO4(s) + 5H2O (l) → CuSO4+5H2O(s) ΔH° = -18.69 kcal
- Enthalpy of Transition: The changes brought about in the enthalpy when one mole of a substance is transformed from one allotropic form to another allotropic form.
C (graphite state) →C(diamond state) ΔH° = 1.9 kJ/mol
- Enthalpy of Sublimation: It is the enthalpy change that occurs when one mole of a solid is converted directly into vapour at a temperature below its melting point.
12(s) —> 12(g) ΔH = 14.9 kcal/mol,
ΔH sublimation = ΔH(fusion) + ΔH(vapourization)
- Enthalpy of Atomisation: The amount of heat required for the formation of one mole of atoms in a gaseous state from its element is known as the heat of atomization of that element.
C(s) → C(9) ΔH = 171.7 kcal/mol
H2(g) → 2H(g) ΔH = 104 kcal
The heat of atomization of hydrogen in this example is 52 kcal/mol.
- Enthalpy or Heat of Solution: The quantity of heat released or absorbed when one mole of solute is dissolved in excess of the solvent, causing further solvent addition to cause no change in heat.
H2SO4+ aqua → H2SO4(aq) ΔH = -20.2 kcal
- Lattice Enthalpy: Lattice enthalpy of an ionic compound is defined as the amount of energy released when one mole of an ionic compound is formed by the interaction of the required number of constituents gaseous cation and gaseous anion.
Laws of Thermochemistry
[Click Here for Sample Questions]
Measuring the heat change in the laboratory is not practical for some reactions. As a result, a traditional technique based on the principle of energy conservation has been proposed, which can be described as follows:
- At any given temperature and pressure, the heat of creation of any compound is equal in size and opposite in sign to the heat of dissociation of that compound.
The enthalpy of production of liquid water from its constituents hydrogen and oxygen, for example, is - 285.830 kJ mol-1, but the enthalpy of dissociation is also 285.830 kJ mol-1. As a result, the procedure can be described by
H2(g) + 1/2 O2(g) → H2O (I), ΔH(298 K) = -285.830 kJ
H2O(l) →H2(g) +1/2 O2(g), ΔH (298 K) = + 285.830 kJ
- Regardless of whether a reaction is performed in one step or multiple steps, the overall enthalpy change is the same. (Hess's Law of Constant Heat Summation) It has been empirically proven and is also a result of the law of energy conservation. It's especially useful for calculating temperatures of processes that are difficult to quantify with calorimetric methods.

Laws of Thermochemistry
Carbon, for example, might be turned into CO2 in a single step.
C(s) + O2(g) →CO2(g) ΔH = -94 kcal
or in two steps
C(s) + 1/2 O2(g) → CO(g) ΔH1 = -26.4 kcal
CO(g) + 1/2 O2(g) → CO2(g) ΔH2 = -67.6 kcal
According to Hess's law:
ΔH = ΔH, + ΔH2
or
- 94 = -26.4 - 67.6
Calorimetry
[Click Here for Previous years Questions]
Calorimetry is a technique for determining how much heat is generated or absorbed during a chemical reaction. It is possible to identify whether a process is exothermic (releases heat) or endothermic (stores heat) by measuring the change in heat (absorbs heat). Calorimetry is also used in everyday life to control metabolic rates in humans and, as a result, to maintain functions like body temperature.
Calorimetry is defined as the transfer of heat from a hotter body to a cooler body with no heat loss to the atmosphere. Calorimetry works on the principle that heat lost by one body equals heat gained by another.
-
Calorimeter:-
A calorimeter is a device that measures the amount of heat produced by a chemical reaction. A calorimeter is made up of a metallic vessel and a stirrer that are both made of the same material (copper or aluminum), and the vessel is enclosed in a wooden jacket to prevent heat loss. Through a small aperture in the outer jacket, a mercury thermometer can be inserted.
-
Calorimetry Important Formulas:-
The heat released by the hot body = Heat absorbed by the cold body.
Q=mcΔT;
where Q= Heat Energy,
m=mass of the body,
c=Specific heat capacity of the body,
ΔT= change in temperature.
Also Read:
Things To Remember
- Thermochemistry is the branch of physical chemistry dealing with heat changes in a reaction.
- System+Surrounding=Universe
- Endothermic and Exothermic are the two types of thermochemical reactions.
- There are several types of enthalpies based on the type of reaction like enthalpy of formation, enthalpy of combustion, enthalpy of neutralization, enthalpy of atomization, etc.
- Calorimetry is defined as the transfer of heat from a hotter body to a cooler body with no heat loss to the atmosphere. Calorimetry works on the principle that heat lost by one body equals heat gained by another.
Sample Questions
Ques. Consider the following hypothetical reactions (2 marks)
A → B, ΔH = 30 kJ
B → C, ΔH = 60 kJ
Using Hess’s law, calculate the change in enthalpy for reaction A→C.
Ans. According to Hess Law, the total enthalpy change is the same regardless of the reaction that takes place in 1 step or 2 steps.
Thus,
ΔH (A → C) = ΔH (A → B) + ΔH (B → C)
ΔH (A → C) = 30+60
ΔH (A → C) = 90 kJ
Ques. Calculate the enthalpy change for the following reaction (2 marks)
H2(g) + 1/2 O2(g) → H2O (g)
If the band energies of H-H, O=O, and O-H bonds are 104, 118, and 111 kcal mol-1 respectively.
Ans. In the given example, energy is absorbed in the breaking of H-H and O=O bonds while it is released in the formation of two O-H bonds. Thus, for the reaction,
ΔH = (104 + ½ x 118) - ( 2 x 111)
= -59 kcal.
Ques. What is the enthalpy of transmission? (2 marks)
Ans. Enthalpy of transmission refers to the changes brought about in the enthalpy when one mole of a substance is transformed from one allotropic form to another allotropic form.
C (graphite state) →C(diamond state) ΔH° = 1.9 kJ/mol
Ques. What is meant by ?H= -95 kJ? (1 mark)
Ans. It means that the system is losing 95kJ of energy while the surroundings are gaining 95kJ of energy.
Ques. From the given data, calculate heat of formation of glucose. (3 marks)
C(s) + O2(g) → CO2(g); ΔH =-395.0kJ
H2(g) + ½ O2(g) → H2O(l); ΔH = -269.4 kJ
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) ΔH = -2900 kJ
Ans. The required equation is
6C(s) + 6H2(g) + 3O2(g) → C6H12O6(s); ΔH =?
With the help of given equations,
ΔH (formation) = [6 x (-395) + 6 x (-269.4)] - (-2900)
= -3986.4 + 2900
= -1086.4 kJ
Hence, the heat of formation of glucose is -1086.4 kJ mol-1.
Ques. What is Calorimetry? State its uses. (2 marks)
Ans. Calorimetry is a technique for determining how much heat is generated or absorbed during a chemical reaction. It is possible to identify whether a process is exothermic or endothermic by measuring the change in heat
Calorimetry is also used in everyday life to control metabolic rates in humans and, as a result, to maintain functions like body temperature.
Ques. Two aqueous solutions at room temperature are mixed in a coffee cup calorimeter. The temperature of the resultant solution falls below room temperature as a result of the reaction. Which of the following statements is TRUE? (2 marks)
A) During the reaction, energy leaves the system.
B) The potential energy of the products is lower than the potential energy of the reactants.
C) This type of experiment yields ΔErxn
D) The mixing is endothermic.
E) The solution possesses unique characteristics that allow it to defy the first and second laws of thermodynamic.
Ans. (D) The mixing is endothermic
Ques. Differentiate between exothermic and endothermic reactions. (3 marks)
Ans.
| Exothermic | Endothermic |
|---|---|
| Any process that causes the system's enthalpy H (or internal energy U) to fall is called an exothermic process. | Any process that causes the system's enthalpy H (or internal energy U) to rise is called an endothermic process. |
| A closed system releases thermal energy to its surroundings throughout this phase, resulting in heat transfer into the surroundings. | A closed system absorbs thermal energy from its surroundings throughout this phase, resulting in heat transfer into the system. |
| It could be a chemical or physical process, such as combustion. | It could be a chemical or physical process, such as dissolving ammonium nitrate in water. |
Do Check Out:






Comments