The equivalent weight of KIO3 in the given reaction is (M=molecular mass) :
2Cr(OH)3 + 4OH + KIO3 → 2CrO42- + 5H2O +KI
The equivalent weight of KIO3 in the given reaction is (M=molecular mass) :
2Cr(OH)3 + 4OH + KIO3 → 2CrO42- + 5H2O +KI
- \(M\)
- \(\frac{M}{2}\)
- \(\frac{M}{6}\)
- \(\frac{M}{8}\)
The Correct Option is C
Approach Solution - 1
The correct answer is option (C): \(\frac{M}{6}\)
The balanced chemical reaction is \(2Cr(OH)_3+OH^−+KIO_3⟶2CrO_4^{2−}+KI+5H_2O\)
The oxidation number of iodine changes from +5 to -1.
The change in the oxidation number is 6.
The equivalent mass of potassium iodate is \(\frac{\text{Molar\,mass\,of\,potassium\,iodate}}{\text{Change\,in\, the \,oxidation\,number\,of\,iodine}}=\frac{253.34}{3}=84.44=\frac{M}{6}\)
Approach Solution -2
(a) KIO3 it acts as an oxidizing agent in the given redox reaction.
(b) There is change in oxidation number of iodine (in reactant) +5 to −1 (in product).
→ KIO3O.S →+1 + x - 6 = +5
→ In KI O.S → x + 1 = -1
Therefore, I has Oxidation no. +5 in KIO3
Also, I has Oxidation no. -1 in KI
change in oxidation number from +5 to -1 :
+4, +3, +2, +1, 0, -1 = 6
Hence,
Equivalent mass of KIO3 = \(\frac{\text{Molar Mass}}{6}\)
So, the correct option is (C) : \(\frac{M}{6}\).
Top Questions on Redox reactions
- At room temperature, disproportionation of an aqueous solution of in situ generated nitrous acid (HNO2) gives the species
- JEE Advanced - 2024
- Chemistry
- Redox reactions
- How many of the following show disproportionation reactions?
H2O2 , Ag, Cu- , K+ , F2 , Cl2 , ClO3-- JEE Main - 2024
- Chemistry
- Redox reactions
- \(aMnO^{-}_4+bS_2O_{3}^-+H_2O\rightarrow xMnO_2+ySO_{4}^-+zOH^-\)
a and y respectively are- KCET - 2023
- Chemistry
- Redox reactions
Oxidation number of Mo in Ammonophosphomolybdate
- JEE Main - 2023
- Chemistry
- Redox reactions
- The number of electrons involved in the reduction of permanganate to manganese dioxide in acidic medium is _______
- JEE Main - 2023
- Chemistry
- Redox reactions
Questions Asked in WBJEE exam
- \(\lim_{x\rightarrow \infty}\){\(x-\sqrt[n]{(x-a_1)(x-a_2)......(x-a_n)}\)} where a1,a2,.....an are positive rational numbers.The limit
- In an experiment on a circuit, as shown in the figure, the voltmeter shows 8V reading. The resistance of the voltmeter is
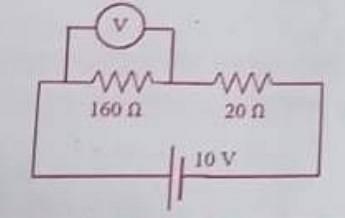
- WBJEE - 2023
- Resistance
- The value of 'a' for which the scaler triple product formed by the vectors \(\vec\alpha=\hat{i}+a\hat{j}+\hat{k}\), \(\vec{\beta}=\hat{j}+a\hat{k}\) and \(\vec{\gamma}=a\hat{i}+\hat{k}\) is maximum, is
- WBJEE - 2023
- Vector Algebra
- A rectangle ABCD has its side parallel to the line y=2x and vertices A,B,D are on y=1,x=1 and x=-1 respectively. The coordinate of C can be
- WBJEE - 2023
- Straight lines
- A missile is fired from the ground level rises x meters vertically upwards in t sec, where \(x=100t-\frac{25}{2}t^2\). the maximum height reached is
- WBJEE - 2023
- Differential equations
Concepts Used:
Redox Reactions
Redox Reaction:
Redox reactions are chemical reactions where oxidation and reduction take place simultaneously. In this type of reaction, there is a gain of electrons for one chemical species while the other loses electrons or simply involves transfer of electrons. The species that loses electrons is oxidized while the one that gains electrons is reduced.
Types of Redox Reactions:
Redox reactions can be differentiated into 4 categories namely combination reactions, decomposition reactions, displacement reactions, and disproportionation reactions. Each is explained separately below:
Combination Reaction:
In this, the molecules combine to form new compounds. For example, when magnesium reacts to nitrogen.
Decomposition Reaction:
Opposite to the combination reaction, here there is a breakdown of compounds to simpler substances. For example, electrolysis of water.
Displacement Reaction:
In this, the more reactive metal will displace the less reactive one in a chemical reaction. The reactivity of an element is represented in a series called the reactivity series (arranged in decreasing order of reactivity) which makes it easier to determine the chemical reaction and its products.
Disproportionation Reaction:
This is a peculiar type of reaction where an element showing a particular oxidation state will be oxidized and reduced simultaneously. Another thing to note is that these reactions will always have an element that can exhibit three oxidation states.



