
Content Curator
Heat Engine is a system that converts heat to mechanical energy. There is an input of heat from a reservoir (hot body) and a discharge of heat (cold body). The system also has a certain amount of heat wastage.
| Table of Content |
Keyterms: Heat Engine, Heat, Energy, Heat transfer, thermal energy, Fuel, coal, gasoline, natural gas, wood, peat, Force, Motion
What is Heat Engine?
[Click Here for Sample Questions]
Heat Engine can be described as a device that converts chemical energy of a fuel into thermal energy which is utilized to do other work. A heat engine will convert the locked energy in the fuel into force and motion.
Fuels used are coal, gasoline, natural gas, wood, and peat. These, when burnt in an engine, release stored energy utilized to power locomotives and factory machinery. Hence the name is heat engine as the burning fuels release the heat stored.
Read more: Joule Thomson Effect
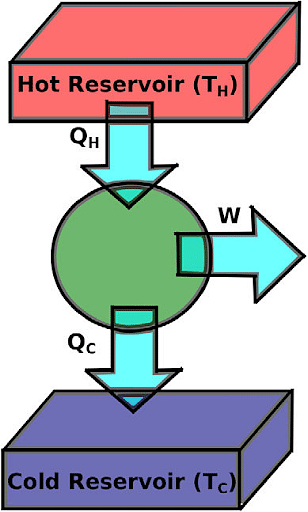
Heat Engine
Functions of Heat Engine
[Click Here for Sample Questions]
A heat engine's job is to transform heat energy into useful mechanical work.
This can be accomplished by taking a working substance. It is first heated to a high temperature before being cooled at a later stage. We can profit from the heat engine in this way. Regardless of the kind of heat engines and cycles, the only fundamental function of the heat system is to transform heat energy into useful mechanical work.
Carnot Engine Video Explanation
Also Read:
| Related Articles | ||
|---|---|---|
| Unit of Foce | Lorentz Force | Unit of Speed |
| Thermal Conductivity | Unit of Conductivity | Difference between heat and temperature |
Types of Heat Engine
[Click Here for Sample Questions]
Heating systems are of two kinds, the first being Internal Combustion engines, and other External Combustion engines.
Internal Combustion Engine
Internal Combustion Engine comprises the burning of fuel within the system, i.e. these engines operate when the fuel is burned in the engine or where the combustion of fossil fuels happens. The internal combustion engine works on Otto Cycle’s principle and the apparatus required during this procedure is generally a piston.
- Piston is responsible for drawing a fuel-air combination into the heat engine, which is the very first step of the process. These pistons scroll vertically within the cylinders of the heat engines. This single upward or downward movement of a piston inside the cylinder is known as a stroke.
- In the second step, the mixture is extremely adiabatically compressed.
- In the third stage, it passes through the ignition process, which dramatically raises the amount of pressure and temperature.
- With its growing temperature, Piston clears the gases once again as the process expands.
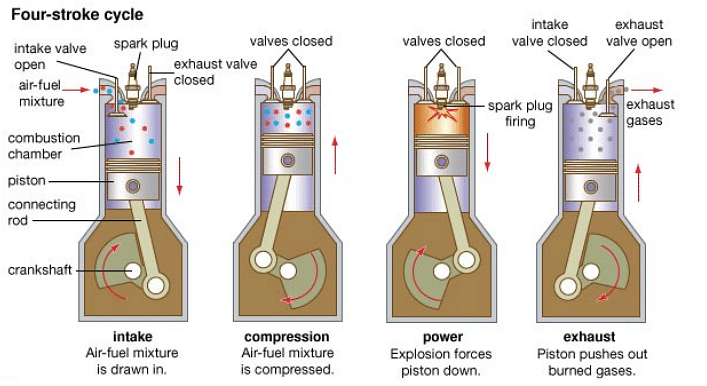
Internal Combustion Engine
External Combustion Engine
These heat engines operate where the fuel is burned outside the engine or where the fuel combustion happens outside the engine. Here, the internal working fluid is heated by external combustion via the engine wall. By expanding and working on the engine's mechanism, this results in fluid generated motion and usable work.
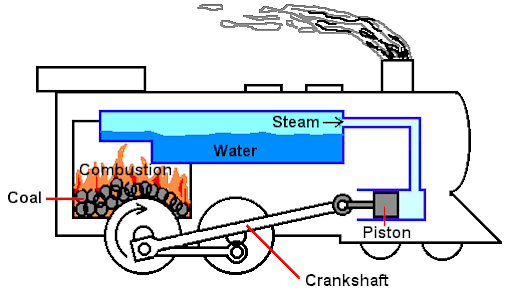
External Combustion Engine
- Like an Internal Combustion engine, here also the piston is responsible for drawing a fuel-air combination into the heat engine and will send it into the first cylinder.
- The gas in the first cylinder is heated to a high temperature, resulting in increased pressure. It is once again gathered by the Piston, which transforms it into work. It is then moved to a different cylinder.
- The gas is distinct from the previous level and also straightforward.
- Now, the cooling operation will be performed on the gas here. The cold reservoir aids in cooling the gas before it is compressed.
- Finally, the compressed gas is returned to the original chamber, where it is collected and converted into productive work by the Piston.
Read More: Thermal Conductivity of Copper
Ideal Heat System
[Click Here for Sample Questions]
An ideal heating system is a system that performs all the processes cyclically. This means wherever it stops again, it finishes from that point itself. An Ideal Heating System contains all of the reversible processes. Carnot Engine is an example of an Ideal Heating System.

Ideal Heat System
Heating Engine: Parts
[Click Here for Sample Questions]
The heating engine's equipment consists of three major components, each of which serves a purpose in turning heat energy into mechanical work.

Heating Engine: Parts
Source (High Temperature)
It is the main component of the heat engine.
It is sometimes referred to as a hot body since the heat engine's primary function is to transform heat into mechanical work. As a result, the source is supplied in the device to collect the heat. It is always kept with an unlimited thermal capacity, allowing it to remain stable even after creating a significant quantity of heat.
Working Object
The amount of heat applied to the hot source will be gathered by this functioning material, which may vary. The function, but on the other hand, is to collect from the hot source and transfer it to the sink.
Sink of Heat (At Lower Temperature)
This is completely contradictory to the first section, which is the source. It just has a limited thermal capability and absorbs heat from the working substance in addition it also aids in the preservation of mechanical work gained from the working substance. However, It does not enable it to be influenced by outside temperatures.
Also Read:
| Related Articles | ||
|---|---|---|
| Geothermal Energy | Thermal Energy | Thermal Conductivity |
| Thermal Stress | Isothermal Process | Decomposition Reaction |
Heat Engine: Mechanism
[Click Here for Sample Questions]
A basic heat engine is made up of a gas contained in a cylinder by a piston. As it is heated, the gas expands, thus pushing the piston. This is not conceivable in a real engine since motion ceases when the gas achieves equilibrium.

Heat Engine: Mechanism
In a functional engine, the piston travels back and forth in cycles. When the gas heats up, the piston travels upward, and when it cools, it moves downward. We can say that to drive the piston forward and backward, a cycle of heating and cooling is required.
Three key highlights of the whole cycle of heat engine:
- Because heat is injected at a relatively high degree, it is referred to as QH.
- Some part of the energy, known as added energy is used to perform the work/tasks.
- Lastly, The left or unused energy is removed relatively at a cold temperature, referred to as QC.
Also Read:
| Related Articles | ||
|---|---|---|
| Types of Motors | Types of Switches | Types of DC Motor |
| Types of Transistors | Types of Friction | Balanced Force |
Efficiency of Heat Engine
[Click Here for Sample Questions]
The efficiency of a heat engine is an important metric because it reflects how much usable work we obtain as an output from the engine when we utilize the same quantity of heat energy as input. Let’s talk in mathematical terms.

Efficiency of Heat Engine
Suppose the engine takes heat (Q1) from a hot reservoir at temperature(T1 )and releases heat (Q2 ) to a cold reservoir at temperature (T2) hereby, delivering work W to the surroundings.
The efficiency of heat engine: η = 1- Q2/Q1
Where η = W/Q1
Note that :
- Q1=heat input in 1 cycle
- Q2=work done in 1 cycle.
Note: There is no such thing as a heat engine with 100% efficiency. There is always a certain loss of heat in the surroundings.
Things to Remember
- Heat Engine is a device that converts heat into mechanical energy.
- Heating Systems are of two types: Internal Combustion Engines and External Combustion Engines.
- Internal Combustion Engine is where fuel is burned within the system. Engines operate when the fuel is burned in the engine.
- External Combustion Engine is where the fuel is burned outside the engine or where the fuel combustion happens outside the engine.
- Carnot Engine is an ideal Heat Engine that works on the principle of a reversible closed thermodynamic cycle. Its four successive operations include Isothermal Expansion, Adiabatic Expansion, Isothermal Compression and Adiabatic Compression
- The heating engine's equipment consists of three major components: Source (High Temperature), Working Object and Sink of Heat (Lower Temperature)
- A basic heat engine consists of gas contained in a cylinder by a piston. As it is heated, the gas expands, thus pushing the piston. In a functional engine, the piston travels back and forth in cycles.
- Heat Engine Efficiency reflects how much usable work is obtained as an output from the engine when the same quantity of heat energy is utilized as input.
Sample Questions
Ques. What is the functionality of the heating system? (1 mark)
Ans. The primary function of a heat engine is to turn heat into work.
It draws heat from a reservoir, then does some work such as moving a piston, lifting weights, and so on before discharging some heat energy into a sink.
Ques. How is the Carnot engine considered an ideal heating system? (1 mark)
Ans. Carnot engine is an ideal heating system because it is a reversible engine operated in a cycle between the hot and cold reservoirs and can have maximum efficiency.
Ques. In the Carnot engine, efficiency is 40%, at hot reservoir temperature T, For efficiency of 50%, what will be the temperature of the hot reservoir? (2 mark)
Ans. From the above formula we can say :
The efficiency, η=workdone/heat input
=W/Q
η=1−T2/T1
where T2= temperature of the sink
and T1= temperature of the hot reservoir
40/100=1−T2/T1
T12/T1=0.6⇒T2=0.6T1
Now, 50/100=1−T2/T1′
⇒T2/T1′=0.5
0.6T1′/T1′=0.5
T1′=0.6/0.5T1⇒T1′=6/5T1
T1=6/5T [∴T1=T′]
Ques. How does an internal combustion engine work? (2 mark)
Ans. The fuel in these heat engines burns within the cylinder. An example of an internal combustion engine is a vehicle engine. Because there is no energy wasted during heat transmission between the boiler and the cylinder, internal combustion engines are more efficient than external combustion engines.
Ques. What is the proportion of stated thermal efficiency to matching air standard cycle efficiency? (1 mark)
Ans. The proportion of stated thermal efficiency to matching air standard cycle efficiency is known as the relative efficiency.
Ques. The efficiency of a heat engine is given as? (1 mark)
Ans. Efficiency of heat engine can be defined as
Net Work Output/Total Heat Input
Ques. Define the process that occurs in a heat engine cycle? (2 mark)
Ans. Three basic processes occur in a heat engine system, namely:
- Steam is condensed in a condenser.
- Work is produced in turbine rotor
- Heat is transferred from the furnace to the boiler.
Ques. Give an example of reciprocating steam engines. (1 mark)
Ans. In some applications, reciprocating engines fueled by compressed air, steam, or other hot gases are still utilized. A steam engine is an example of one.
Ques. What is the combustion in a compression ignition engine? (1 mark)
Ans. In an ignition engine, heterogeneous combustion takes place.
Ques. During a cyclic process, a heat engine absorbs 500 J of heat from a hot reservoir, does work, and ejects an amount of heat 300 J into the surroundings (cold reservoir). Calculate the efficiency of the heat engine? (2 mark)
Ans. The efficiency of the heat engine is given by:
n = 1 - QL/ QH
n=1-300/500
= 1-3/5
n = 1 - 0.6 = 0.4
μ = 1 – 0.6 = 0.4
Thus, efficiency of the heat engine is 40%, ie, only 40% of the input heat is converted to work.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Do Check Out:





Comments