Question:
Which one of the following is the correct relation between Cp and Cv for one mole of an ideal gas? (R is molar gas constant)
Which one of the following is the correct relation between Cp and Cv for one mole of an ideal gas? (R is molar gas constant)
Updated On: Sep 19, 2024
- Cp=Cv-R
- Cp=Cv+R
- Cp=R-Cv
- Cp=Cv×R
- \(C_P=\frac{C_V}{R}\)
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
The correct option is (B) : Cp=Cv+R
Was this answer helpful?
0
1
Top Questions on Thermodynamics
- A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
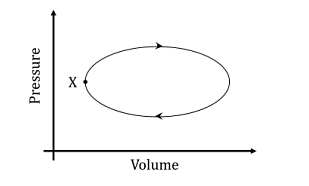
The correct statement about this process is- IIT JAM CY - 2024
- Physical Chemistry
- Thermodynamics
- A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is :
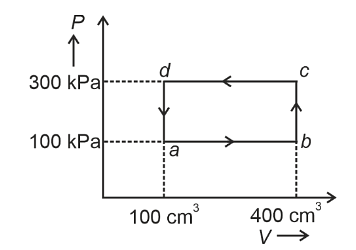
- NEET (UG) - 2024
- Physics
- Thermodynamics
- Which of the following statement is incorrect?
- JEE Main - 2024
- Chemistry
- Thermodynamics
- The enthalpy change for the reaction
C(𝑔) +\( \frac{1}{2}\) O2(𝑔)→CO(𝑔) is _______ kJ per mole of CO(𝑔) produced.
(rounded off to one decimal place)
[Given:
C(𝑔)+O2(𝑔) → CO2(𝑔), ΔHrxn = −393.5 kJ per mole of CO2(𝑔) produced
CO2(𝑔) → CO(𝑔) +\( \frac{1}{2}\) O2(𝑔), ΔHrxn = 283.0 kJ per mole of CO(𝑔) produced]- IIT JAM CY - 2024
- Physical Chemistry
- Thermodynamics
- Identify the reaction for which, at equilibrium, a change in the volume of the closed reaction vessel at a constant temperature will not affect the extent of the reaction
- IIT JAM CY - 2024
- Physical Chemistry
- Thermodynamics
View More Questions
Questions Asked in KEAM exam
- Let \(k\) be a real number such that \(\sin \dfrac{3π}{14} \cos \dfrac{3π}{14} = k \cos \dfrac{π}{14}\).Then the value of \(4k\) is
- KEAM - 2023
- Trigonometry
- Suppose \(A=\begin{bmatrix} a_1 & b_1 & c_1 \\ a_2 & b_2 & c_2 \\ a_3 & b_3 & c_3\end{bmatrix}\) is an adjoint of the matrix \(\begin{bmatrix} 1 & 3 & 3 \\ 1 & 4 & 3 \\ 1 & 3 & 4\end{bmatrix}\). Then the value of \(\frac{a_!+b_2+c_3}{b_1a_2}\) is
- KEAM - 2023
- Matrices
- Let \(f:R→R\) be a function defined by
\(f(x) = \begin{cases} 3e^x & \quad \text {if}\ {x<0}\\ x^2+3x+3 & \quad \text {if}\ 0≤x<1 \\ x^2-3x-3 & \quad \text {if} \ x≥1\end{cases}\)- KEAM - 2023
- Limit and Continuity
The angle of minimum deviation for a prism of apex angle 60° and refractive index of \(\sqrt{2}\) is:
- KEAM - 2023
- Ray optics and optical instruments
- A thin particle moves from \((0,1)\) and gets reflected upon hitting the x-axis at \((√3,0)\). Then the slope of the reflected line is ?
- KEAM - 2023
- Slope of a line
View More Questions
KEAM Notification
 JCECEB has released Seat Matrix of Online Counselling, Check HereSep 19, 2024
JCECEB has released Seat Matrix of Online Counselling, Check HereSep 19, 2024The Seat Allotment for 3rd round is scheduled for Sep 22. Provisional seat allotment (3rd round), Document Verification & Admission in the Concerned Institute is scheduled for Sep 23 - 30, 2024



