Question:
A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is :
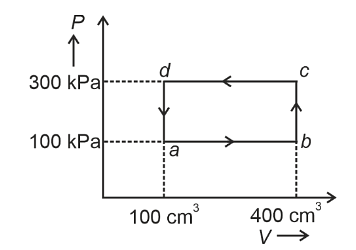
A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is :


Updated On: Oct 27, 2024
- Zero
- 30 J
- –90 J
- –60 J
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
The correct option is (A) :Zero.
Was this answer helpful?
1
3
Top Questions on Thermodynamics
- A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
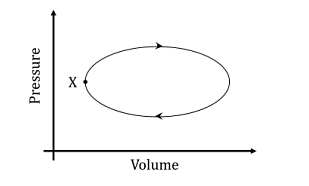
The correct statement about this process is- IIT JAM CY - 2024
- Physical Chemistry
- Thermodynamics
- One mole of an ideal monoatomic gas, initially at temperature 𝑇𝑜 is expanded from an initial volume 𝑉𝑜 to 2.5𝑉𝑜. Which of the following statements is(are) correct?
(R is the ideal gas constant)- IIT JAM - 2024
- Physics
- Thermodynamics
- The enthalpy change for the reaction
C(𝑔) +\( \frac{1}{2}\) O2(𝑔)→CO(𝑔) is _______ kJ per mole of CO(𝑔) produced.
(rounded off to one decimal place)
[Given:
C(𝑔)+O2(𝑔) → CO2(𝑔), ΔHrxn = −393.5 kJ per mole of CO2(𝑔) produced
CO2(𝑔) → CO(𝑔) +\( \frac{1}{2}\) O2(𝑔), ΔHrxn = 283.0 kJ per mole of CO(𝑔) produced]- IIT JAM CY - 2024
- Physical Chemistry
- Thermodynamics
- Identify the reaction for which, at equilibrium, a change in the volume of the closed reaction vessel at a constant temperature will not affect the extent of the reaction
- IIT JAM CY - 2024
- Physical Chemistry
- Thermodynamics
- Which of the following statement is incorrect?
- JEE Main - 2024
- Chemistry
- Thermodynamics
View More Questions
Questions Asked in NEET exam
- In a Vernier caliper, \(N+1\) divisions of vernier scale coincide with \(N\) divisions of main scale. If 1 MSD represents 0.1 mm, the vernier constant (in cm) is:
- NEET (UG) - 2024
- Units, Dimensions and Measurements
- A tightly wound \(100\) turns coil of radius \(10 cm\) carries a current of \( 7 A\). The magnitude of the magnetic field at the centre of the coil is (Take permeability of free space as \(4\pi×10^{-7}\) SI units):
- NEET (UG) - 2024
- Magnetic Field
- Consider the following statements A and B and identify the correct answer:

A. For a solar-cell, the I-V characteristics lies in the IV quadrant of the given graph.
B. In a reverse biased pn junction diode, the current measured in (µA), is due to majority charge carriers.- NEET (UG) - 2024
- P-N Junction
- Given below are two statements :
Statement I : In the nephron, the descending limb of loop of Henle is impermeable to water and permeable to electrolytes.
Statement II : The proximal convoluted tubule is lined by simple columnar brush border epithelium and increases the surface area for reabsorption.
In the light of the above statements, choose the correct answer from the option given below :- NEET (UG) - 2024
- Nephrons
- The maximum elongation of a steel wire of 1 m length if the elastic limit of steel and its Young’s modulus, respectively, are 8 × 108 N m–2 and 2 × 1011 N m–2, is :
- NEET (UG) - 2024
- Youngs double slit experiment
View More Questions



