Question:
Identify the reaction for which, at equilibrium, a change in the volume of the closed reaction vessel at a constant temperature will not affect the extent of the reaction
Identify the reaction for which, at equilibrium, a change in the volume of the closed reaction vessel at a constant temperature will not affect the extent of the reaction
Updated On: Oct 1, 2024
- CaCO3 (𝑠) ⇌ CaO(𝑠) + CO2(𝑔)
- H2 (𝑔) + I2 (𝑔) ⇌ 2HI(𝑔)
- 2NO2 (𝑔) ⇌ N2O4 (𝑔)
- CO2 (𝑠) ⇌ CO2 (𝑔)
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
The correct option is (B): H2 (𝑔) + I2 (𝑔) ⇌ 2HI(𝑔)
Was this answer helpful?
0
0
Top Questions on Thermodynamics
- The enthalpy of formation of ethane (\( \text{C}_2\text{H}_6 \)) from ethylene by addition of hydrogen,
where the bond energies of \( \text{C} - \text{H} \), \( \text{C} - \text{C} \), \( \text{H} - \text{H} \) are \( 414 \, \text{kJ} \), \( 347 \, \text{kJ} \), \( 615 \, \text{kJ} \), and \( 435 \, \text{kJ} \) respectively, is _________ \( \text{kJ} \).- JEE Main - 2024
- Chemistry
- Thermodynamics
- Three moles of an ideal gas are compressed isothermally from 60 L to 20 L using constant pressure of 5 atm. Heat exchange Q for the compression is – ____ Lit. atm.
- JEE Main - 2024
- Chemistry
- Thermodynamics
- A sample of gas at temperature \( T \) is adiabatically expanded to double its volume. Adiabatic constant for the gas is \( \gamma = \frac{3}{2} \). The work done by the gas in the process is (\( \mu = 1 \) mole):
- JEE Main - 2024
- Physics
- Thermodynamics

\([ \text{Given: Absolute temperature} = ^\circ \text{C} + 273.15, \, R = 0.08206 \, \text{L atm mol}^{-1} \text{K}^{-1} ]\)- JEE Main - 2024
- Chemistry
- Thermodynamics
- A mixture of one mole of a monoatomic gas and one mole of a diatomic gas (rigid) are kept at room temperature (\(27^\circ \text{C}\)). The ratio of specific heat of gases at constant volume respectively is:
- JEE Main - 2024
- Physics
- Thermodynamics
View More Questions
Questions Asked in IIT JAM CY exam
- A salt QCl of a certain metal Q is electrolyzed to its elements. 40 g of metal Q is formed at an electrode. The volume of Cl2 formed at the other electrode at 1 atm pressure and 298 K is _______ litres. (rounded off to one decimal place)
[Given: The gas constant 𝑅 = 0.082 L atm mol−1 K−1 , the molar mass of Q is 40 g mol−1 and Cl2 is assumed to be an ideal gas]- IIT JAM CY - 2024
- Electrochemistry
- Exhaustive hydrogenation of the following compound
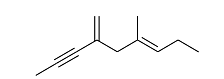
under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is _______.- IIT JAM CY - 2024
- Stereochemistry
- In the cell reaction
P+(𝑎𝑞)+Q(𝑠)→P(𝑠)+Q+(𝑎𝑞)
the EMF of the cell, 𝐸𝑐𝑒𝑙𝑙 is zero. The standard EMF of the cell, 𝐸𝑜𝑐𝑒𝑙𝑙 is
[Given:
Activities of all solids are unity.
Activity of P+(𝑎𝑞) is 2 M. Activity of Q+(𝑎𝑞) is 1 M.
𝑅 = universal gas constant; 𝑇 = temperature; 𝐹 = Faraday constant]- IIT JAM CY - 2024
- Electrochemistry
- The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:
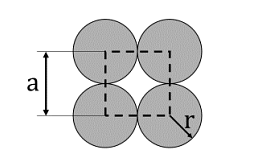
The percentage (%) area occupied by the grey circles (of radius r) inside the unit cell is _______. (rounded off to the nearest integer)- IIT JAM CY - 2024
- Solid State
- The ratio of osmotic pressures of aqueous solutions of 0.01 M BaCl2 to 0.005 M NaCl is
[Given: Both compounds dissociate completely in water]- IIT JAM CY - 2024
- Chemical equilibria
View More Questions



