Question:
With respect to graphite and diamond, which of the statement(s) given below is/are correct?
With respect to graphite and diamond, which of the statement(s) given below is/are correct?
Updated On: Jun 14, 2022
- Graphite is harder than diamond
- Graphite has higher electrical conductivity than diamond.
- Graphite has higher thermal conductivity than diamond.
- Graphite has higher C- C bond order than diamond
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
Diamond has a three-dimensional network structure, a hard substance where graphite is soft due to layered structure.In graphite, only three valence electrons are involved in bonding and one electron remain free giving electrical conductivity. In diamond, all the four valence electrons are covalently bonded hence, insulator. Diamond is better thermal conductor than graphite. Electrical conductivity is due to availability of free electrons, thermal conduction is due to transfer of thermal vibrational energy from one atom to another atom. A compact and precisely aligned crystals like diamond thus facilitate better movement of heat. In graphite C- C bond acquire some double bond character, hence, higher bond order than in diamond.
Was this answer helpful?
0
0
Top Questions on p -Block Elements
- Arrange the following according to their decreasing oxidizing power : BrO4-, IO4-, CIO4-
- JEE Main - 2024
- Chemistry
- p -Block Elements
- Match List-I with List – II :
List-I (Oxoacids of Sulphur) List-II (Bonds) A Peroxodisulphuric acid I Two S–OH, Four S=O, One S–O–S B Sulphuric acid II Two S–OH, One S=O C Pyrosulphuric acid III Two S–OH, Four S=O, One S–O–O–S D Sulphurous acid IV Two S–OH, Two S=O Choose the correct answer from the options given below.- NEET (UG) - 2023
- Chemistry
- p -Block Elements
- Taking stability as the factor, which one of the following represents correct relationship?
- NEET (UG) - 2023
- Chemistry
- p -Block Elements
- Match List I with List II
LIST I LIST II A Chlorophyll 1 \(Na _2 CO _3\) B Soda ash 2 \(CaSO _4\) C Dentistry, Ornamental work 3 \(Mg ^{2+}\) D Used in white washing 4 \(Ca ( OH )_2\) - JEE Main - 2023
- Chemistry
- p -Block Elements
Compound A reacts with $NH _4 Cl$ and forms a compound B.
Compound B reacts with $H _2 O$ and excess of $CO _2$ to form compound $C$ which on passing through or reaction with saturated $NaCl$ solution forms sodium hydrogen carbonate Compound $A , B$ and $C$, are respectively.
- JEE Main - 2023
- Chemistry
- p -Block Elements
View More Questions
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
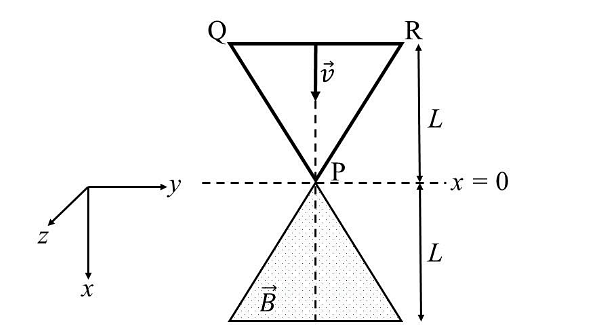
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
View More Questions
Concepts Used:
P-Block Elements
- P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
- P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
P block elements consist of:
- Group 13 Elements: Boron family
- Group 14 Elements: Carbon family
- Group 15 Elements: Nitrogen family
- Group 16 Elements: Oxygen family
- Group 17 Elements: Fluorine family
- Group 18 Elements: Neon family



