Question:
Which one of the following statements is correct in the context of thermodynamics?
Which one of the following statements is correct in the context of thermodynamics?
Updated On: Jul 18, 2024
- In a closed system, neither mass nor energy is transferred across the system boundary
- In a closed system, both mass and energy can be transferred across the system boundary
- The total energy of the system is the sum of kinetic and potential energies
- In a closed system, only energy can be transferred across the system boundary and not mass
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
The correct option is (D):In a closed system, only energy can be transferred across the system boundary and not mass
Was this answer helpful?
0
0
Top Questions on Laws of thermodynamics
- The quantity which changes with temperature:
- JEE Main - 2024
- Chemistry
- Laws of thermodynamics
- Consider the following volume−temperature (V−T) diagram for the expansion of 5 moles of an ideal monoatomic gas.
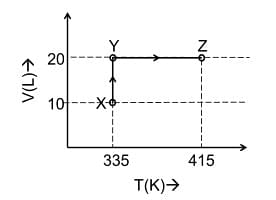
Considering only P-V work is involved, the total change in enthalpy (in Joule) for the transformation of state in the sequence X→Y→Z is ______.
[Use the given data: Molar heat capacity of the gas for the given temperature range, CV,m = 12 J K−1mol−1 and gas constant, R=8.3 JK−1 mol−1]- JEE Advanced - 2024
- Chemistry
- Laws of thermodynamics
- Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A: In an Ellingham diagram, the oxidation of carbon to carbon monoxide shows a negative slope with respect to temperature
Reason R: CO tends to get decomposed at higher temperature
In the light of the above statements, choose the correct answer from the options given below- JEE Main - 2023
- Chemistry
- Laws of thermodynamics
- A certain amount of gas of volume $V$ at $27^{\circ} C$ temperature and pressure $2 \times 10^7 Nm ^{-2}$ expands isothermally until its volume gets doubled Later it expands adiabatically until its volume gets redoubled The final pressure of the gas will be (Use $\gamma=15$ )
- JEE Main - 2023
- Physics
- Laws of thermodynamics
- How many reactions are non-spontaneous at 300 K. For independent reaction ΔH & ΔS values are given.
- JEE Main - 2023
- Chemistry
- Laws of thermodynamics
View More Questions
Questions Asked in GATE BT exam
- The transfer function of a process is \( G(s) = \frac{K_p}{T_p s + 1} \), where \( K_p \) is the gain and \( T_p \) is the time constant. This is a _____ process.
- GATE BT - 2024
- Pressure, temperature and flow measurement devices
- Under which of the following conditions, a mammalian somatic cell fails to undergo mitosis during cell cycle?
- GATE BT - 2024
- Cell cycle and cell growth control
- Which of the following is/are considered as biotic elicitor(s) in plant cell culture?
- GATE BT - 2024
- Anchorage and non-anchorage dependent cell culture
- Which of the following statements is/are correct about an uncompetitive inhibitor of an enzyme?
- GATE BT - 2024
- Enzyme inhibition
- Which of the following conditions induce(s) the expression of B-galactosidase gene in the lac operon?
- GATE BT - 2024
- Concepts and regulation of metabolism of carbohydrates
View More Questions



