Question:
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A: In an Ellingham diagram, the oxidation of carbon to carbon monoxide shows a negative slope with respect to temperature
Reason R: CO tends to get decomposed at higher temperature
In the light of the above statements, choose the correct answer from the options given below
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A: In an Ellingham diagram, the oxidation of carbon to carbon monoxide shows a negative slope with respect to temperature
Reason R: CO tends to get decomposed at higher temperature
In the light of the above statements, choose the correct answer from the options given below
Assertion A: In an Ellingham diagram, the oxidation of carbon to carbon monoxide shows a negative slope with respect to temperature
Reason R: CO tends to get decomposed at higher temperature
In the light of the above statements, choose the correct answer from the options given below
Updated On: Sep 24, 2024
- A is correct but $R$ is not correct
- $A$ is not correct but $R$ is correct
- Both $A$ and $R$ are correct and $R$ is the correct explanation of $A$
- Both $A$ and $R$ are correct but $R$ is NOT the correct explanation of $A$
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
Correct answer is (a): A is correct but R is not correct
is ; thus slope is negative
As temperature increases becomes more negative thus it has lower tendency to get decomposed.
is ; thus slope is negative
As temperature increases becomes more negative thus it has lower tendency to get decomposed.
Was this answer helpful?
1
0
Top Questions on Laws of thermodynamics
- Which one of the following statements is correct in the context of thermodynamics?
- GATE BT - 2024
- Fundamentals of Biological Engineering
- Laws of thermodynamics
- The quantity which changes with temperature:
- JEE Main - 2024
- Chemistry
- Laws of thermodynamics
- Consider the following volume−temperature (V−T) diagram for the expansion of 5 moles of an ideal monoatomic gas.
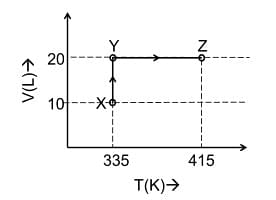
Considering only P-V work is involved, the total change in enthalpy (in Joule) for the transformation of state in the sequence X→Y→Z is ______.
[Use the given data: Molar heat capacity of the gas for the given temperature range, CV,m = 12 J K−1mol−1 and gas constant, R=8.3 JK−1 mol−1]- JEE Advanced - 2024
- Chemistry
- Laws of thermodynamics
- A certain amount of gas of volume $V$ at $27^{\circ} C$ temperature and pressure $2 \times 10^7 Nm ^{-2}$ expands isothermally until its volume gets doubled Later it expands adiabatically until its volume gets redoubled The final pressure of the gas will be (Use $\gamma=15$ )
- JEE Main - 2023
- Physics
- Laws of thermodynamics
- How many reactions are non-spontaneous at 300 K. For independent reaction ΔH & ΔS values are given.
- JEE Main - 2023
- Chemistry
- Laws of thermodynamics
View More Questions
Questions Asked in JEE Main exam
- Find the acceleration of \(2\) \(kg\) block shown in the diagram (neglect friction)

- JEE Main - 2024
- Acceleration
- If \(|2A|^3 =21\) and \(\begin{bmatrix} 1 & 0 & 0 \\[0.3em] 0 & α & β \\[0.3em] 0 & β & α \end{bmatrix}\), then a is (if \(α,β∈I\))
- JEE Main - 2024
- Matrices
- Let \(α\) and \(β\) the roots of equation \(px^2 + qx - r = 0\), where \(P≠ 0\). If \(p,q,r\) be the consecutive term of non constant G.P and \(\frac{1}{α} + \frac{1}{β} = \frac{3}{4}\) then the value of \((α - β)^2\) is:
- JEE Main - 2024
- Geometric Progression
- Two lines \(L_1 \;\& \;L_2\) passing through origin trisecting the line segment intercepted by the line \(4x + 5y = 20\) between the coordinate axes. Then the tangent of angle between the lines \(L_1\) and \(L_2\) is
- JEE Main - 2024
- Tangents and Normals
- Rank of the word 'GTWENTY' in dictionary is _____ .
- JEE Main - 2024
- permutations and combinations
View More Questions
Concepts Used:
General Principles and Processes of Isolation of Elements
What are Ores and Minerals?
Minerals are the naturally occurring, homogeneous inorganic solid substances. They are having a definite chemical composition and crystalline structure, hardness and color. For example, copper pyrite, calamine, etc.

Impurities in an ore are called gauge. The removal of a gauge from the ore is called concentration ore.
Several steps are involved in the extraction of pure metal from ores. Major steps are as follows –
- Concentration of the ore
- Isolation of the metal from its concentrated ore
- Purification of the metal



