Question:
The bond length of CO is 113 pm and its dipole moment (𝜇⃗) is 0.1 D. The charge (in units of electronic charge) on carbon in the CO molecule including its sign is (rounded off to three decimal places)
[Given: charge of electron = 1.602×10−19 C; 1 D=3.336 × 10−30 Cm]
The bond length of CO is 113 pm and its dipole moment (𝜇⃗) is 0.1 D. The charge (in units of electronic charge) on carbon in the CO molecule including its sign is (rounded off to three decimal places)
[Given: charge of electron = 1.602×10−19 C; 1 D=3.336 × 10−30 Cm]
[Given: charge of electron = 1.602×10−19 C; 1 D=3.336 × 10−30 Cm]
Updated On: Oct 1, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: -0.017 - -0.019
Solution and Explanation
The correct answer is: -0.019) to 0.017 (approx)
Was this answer helpful?
0
0
Top Questions on Chemical bonding and molecular structure
- Number of complexes from the following with even number of unpaired "d" electrons is _____. \[ [\text{V}(\text{H}_2\text{O})_6]^{3+}, \, [\text{Cr}(\text{H}_2\text{O})_6]^{2+}, \, [\text{Fe}(\text{H}_2\text{O})_6]^{3+}, \, [\text{Ni}(\text{H}_2\text{O})_6]^{3+}, \, [\text{Cu}(\text{H}_2\text{O})_6]^{2+}\] [Given atomic numbers: \( \text{V} = 23, \, \text{Cr} = 24, \, \text{Fe} = 26, \, \text{Ni} = 28, \, \text{Cu} = 29 \)]
- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- Which one of the following molecules has maximum dipole moment ?
- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- Number of molecules/ions from the following in which the central atom is involved in sp3 hybridization is ________.\( \text{NO}_3^-, \, \text{BCl}_3, \, \text{ClO}_2^-, \, \text{ClO}_3 \)
- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- The total number of 'sigma' and 'Pi' bonds in 2-oxohex-4-ynoic acid is____.
- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- Number of molecules from the following which are exceptions to octet rule is ____.
\(\text{CO}_2, \, \text{NO}_2, \, \text{H}_2\text{SO}_4, \, \text{BF}_3, \, \text{CH}_4, \, \text{SiF}_4, \, \text{ClO}_2, \, \text{PCl}_5, \, \text{BeF}_2, \, \text{C}_2\text{H}_6, \, \text{CHCl}_3, \, \text{CBr}_4\)- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
View More Questions
Questions Asked in IIT JAM CY exam
- A salt QCl of a certain metal Q is electrolyzed to its elements. 40 g of metal Q is formed at an electrode. The volume of Cl2 formed at the other electrode at 1 atm pressure and 298 K is _______ litres. (rounded off to one decimal place)
[Given: The gas constant 𝑅 = 0.082 L atm mol−1 K−1 , the molar mass of Q is 40 g mol−1 and Cl2 is assumed to be an ideal gas]- IIT JAM CY - 2024
- Electrochemistry
- Exhaustive hydrogenation of the following compound
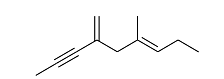
under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is _______.- IIT JAM CY - 2024
- Stereochemistry
- In the cell reaction
P+(𝑎𝑞)+Q(𝑠)→P(𝑠)+Q+(𝑎𝑞)
the EMF of the cell, 𝐸𝑐𝑒𝑙𝑙 is zero. The standard EMF of the cell, 𝐸𝑜𝑐𝑒𝑙𝑙 is
[Given:
Activities of all solids are unity.
Activity of P+(𝑎𝑞) is 2 M. Activity of Q+(𝑎𝑞) is 1 M.
𝑅 = universal gas constant; 𝑇 = temperature; 𝐹 = Faraday constant]- IIT JAM CY - 2024
- Electrochemistry
- A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
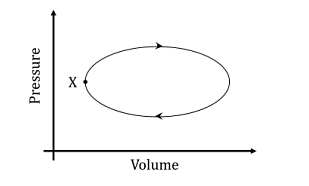
The correct statement about this process is- IIT JAM CY - 2024
- Thermodynamics
- The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:
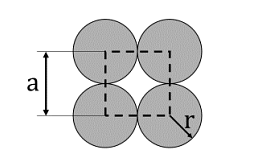
The percentage (%) area occupied by the grey circles (of radius r) inside the unit cell is _______. (rounded off to the nearest integer)- IIT JAM CY - 2024
- Solid State
View More Questions



