Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A: Helium is used to dilute oxygen in diving apparatus.
Reason R: Helium has high solubility in O2.
In the light of the above statements, choose the correct answer from the options given below.
Assertion A: Helium is used to dilute oxygen in diving apparatus.
Reason R: Helium has high solubility in O2.
In the light of the above statements, choose the correct answer from the options given below.
- Both A and R are true but R is NOT the correct explanation of A.
A is true but R is false.
A is false but R is true.
- Both A and R are true and R is the correct explanation of A.
The Correct Option is B
Approach Solution - 1
The correct option is (B): A is true but R is false.
Explanation:
Assertion A states that helium is used to dilute oxygen in the diving apparatus. This is true. Helium is often used as a diluent gas in diving mixtures because it has a low density and does not react with other gases. Adding helium to the breathing gas mixture, it helps reduce the density and minimize the risk of nitrogen narcosis, which can occur at greater depths when breathing air with high nitrogen content.
Reason R states that helium has a high solubility in O2. This statement is incorrect. In reality, helium has low solubility in oxygen. Unlike nitrogen, helium does not dissolve significantly in oxygen under normal conditions. This low solubility is one of the reasons why helium is preferred over nitrogen in diving mixtures.
Since Reason R is incorrect, the correct answer would be option (b), where Assertion is correct.
Approach Solution -2
The correct option is (B): A is true but R is false.
Explanation:
For Assertion - Helium and Oxygen mixture are used in diving apparatus for maintaining the normal air pressure inside the suit making it more comfortable for the divers. Helium is used here to reduce the risk of developing Nitrogen narcosis which can lead to higher Nitrogen concentration in the blood by dissolving and turning out to be fatal. Helium gets less dissolved thus reducing its density and preventing such dangerous scenarios while diving.
For Reason - But the mentioned reason here isn’t right as Helium is very less soluble in oxygen and for that reason only it is used instead of Nitrogen to prevent any narcosis condition during the diving by not causing excessive pressure.
For that reason the Assertion is true but the Reason is false. So, the option B is correct.
Approach Solution -3
Reasons why Assertion A “Helium is used to dilute oxygen in diving apparatus.” is correct:
- A mixture of Oxygen and Helium is used that gives the same partial pressure of O2 as in normal at 1 atm for diving apparatus.
- Due to greater solubility and high partial pressure, Nitrogen (N2) gets dissolved in blood and form bubbles which is very painful for the divers.
- Both Nitrogen and Oxygen can induce a situation similar to drunkenness but adding Helium to the mixture reduces this.
- Therefore, Nitrogen is replaced by Helium as it is much less soluble in biological fluids.
Helium has very low solubility in oxygen. In fact, helium is one of the gases with the lowest solubility in blood and other biological fluids.
Top Questions on p -Block Elements
- $\textbf{Choose the correct statements about the hydrides of group 15 elements.}$
A. The stability of the hydrides decreases in the order \(\text{NH}_3 > \text{PH}_3 > \text{AsH}_3 > \text{SbH}_3 > \text{BiH}_3\)
B. The reducing ability of the hydrides increases in the order \(\text{NH}_3 < \text{PH}_3 < \text{AsH}_3 < \text{SbH}_3 < \text{BiH}_3\)
C. Among the hydrides, \(\text{NH}_3\) is a strong reducing agent while \(\text{BiH}_3\) is a mild reducing agent.
D. The basicity of the hydrides increases in the order \(\text{NH}_3 < \text{PH}_3 < \text{AsH}_3 < \text{SbH}_3 < \text{BiH}_3\)
Choose the most appropriate from the option given below:- JEE Main - 2024
- Chemistry
- p -Block Elements
- Identify the correct statements about p-block elements and their compounds.
(A) Non metals have higher electronegativity than metals.
(B) Non metals have lower ionisation enthalpy than metals.
(C) Compounds formed between highly reactive nonmetals and highly reactive metals are generally ionic.
(D) The non-metal oxides are generally basic in nature.
(E) The metal oxides are generally acidic or neutral in nature.- JEE Main - 2024
- Chemistry
- p -Block Elements
- $\textbf{Reduction potential of ions are given below:}$
\[ \begin{array}{ccc} \text{ClO}_4^- & \text{IO}_4^- & \text{BrO}_4^- \\ E^\circ = 1.19 \, \text{V} & E^\circ = 1.65 \, \text{V} & E^\circ = 1.74 \, \text{V} \\ \end{array} \]
The correct order of their oxidising power is:- JEE Main - 2024
- Chemistry
- p -Block Elements
The number of oxygen atoms present in chemical formula of fuming sulphuric acid is _______.
- JEE Main - 2024
- Chemistry
- p -Block Elements
- Taking stability as the factor, which one of the following represents correct relationship?
- NEET (UG) - 2023
- Chemistry
- p -Block Elements
Questions Asked in NEET exam
- Which one of the following is not a criterion for classification of fungi?
- NEET (UG) - 2024
- Kingdom Fungi
- A parallel plate capacitor is charged by connecting it to a battery through a resistor. If I is the current in the circuit, then in the gap between the plates :
- NEET (UG) - 2024
- Capacitors and Capacitance
- Match List I with List II.Choose the correct answer from the options given below:
List I
(Spectral Lines of Hydrogen for transitions from)List II
(Wavelength (nm))A. n2 = 3 to n1 = 2 I. 410.2 B. n2 = 4 to n1 = 2 II. 434.1 C. n2 = 5 to n1 = 2 III. 656.3 D. n2 = 6 to n1 = 2 IV. 486.1 - NEET (UG) - 2024
- Bohr’s Model for Hydrogen Atom
- Match List I with List IIChoose the correct answer from the options given below
List-I List-II A Rhizopus I Mushroom B Ustilago II Smut fungus C Puccinia III Bread mould D Agaricus IV Rust fungus - NEET (UG) - 2024
- Nomenclature and taxonomy
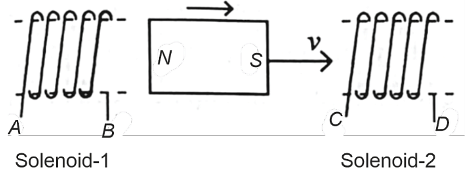
In the above diagram, a strong bar magnet is moving towards solenoid-2 from solenoid-1. The direction of induced current in solenoid-1 and that in solenoid-2, respectively, are through the directions :- NEET (UG) - 2024
- Solenoids and Toroids
Concepts Used:
P-Block Elements
- P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
- P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
P block elements consist of:
- Group 13 Elements: Boron family
- Group 14 Elements: Carbon family
- Group 15 Elements: Nitrogen family
- Group 16 Elements: Oxygen family
- Group 17 Elements: Fluorine family
- Group 18 Elements: Neon family