
Content Curator
SRMJEEE 2017 Physics Question paper with answer key pdf is available for download. The exam was successfully organized by SRM University. The question paper comprised a total of 25 questions.
SRMJEEE 2017 Physics Question Paper with Answer Key PDFs
| SRMJEEE 2017 Physics Question Paper PDF | SRMJEEE 2017 Physics Answer Key PDF |
|---|---|
| Download PDF | Available Soon |












SRMJEEE Previous Year Question Paper with Answer Key PDFs
Similar Exam Question Papers
SRMJEEE Questions
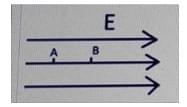
2. If VA and VVB are the potentials of the two points A and B, what will be the correct relation between the electric field and the potential difference between the points? The distance between the points is d.

- VA- VB = \(\frac{d}{E}\)
- VA-VB = Ed
- VB- VA = Ed
- VA-VA = \(\frac{E}{d}\)
3. An ideal diode presents a(n)______ when reversed-biased and a(n) __________ when forward-biased.
- Open, short
- Short, open
- Open, open
- Short, short
4. The molar conductivities at infinite dilution for Na2SO4,K2S04,KCl, HCl and HCOONa at 300K are 260, 308, 150, 426, and 105 S cm2 mol-1, respectively. What will be A+m for formic acid in the same unit?
The molar conductivities at infinite dilution for Na2SO4,K2S04,KCl, HCl and HCOONa at 300K are 260, 308, 150, 426, and 105 S cm2 mol-1, respectively. What will be A+m for formic acid in the same unit?
429
405
531
381
5. Electrophilic halogenation of phenol does not require catalyst because
Electrophilic halogenation of phenol does not require catalyst because
Phenol is electron rich
Oxygen in phenol is more reactive
Phenol is electron deficient as oxygen is the second is the second most electronegative element
Phenol is a planar molecule
6. Which of the following statement is true for aqueous solution of 0.1 M urea, 0.2 M glucose nad 0.3 M sucrose
Which of the following statement is true for aqueous solution of 0.1 M urea, 0.2 M glucose nad 0.3 M sucrose
The elevation in boiling point is the highest for urea.
The vapour pressure and boiling point are the lowest for urea.
The depression in freezing point is the highest for urea.
The vapour pressure and freezing point are the the lowest for urea.



.png?h=160&w=320&mode=stretch)
.png?h=160&w=320&mode=stretch)
.png?h=160&w=320&mode=stretch)
.png?h=160&w=320&mode=stretch)



Comments