Question:
Which of the following statemnt is incorect for physisorption?
Which of the following statemnt is incorect for physisorption?
Updated On: Apr 30, 2024
- it arises because of Van der Waal's force
- it is reversibble in nature
- it is not specific in nature
- High temperature is favorable for adsorption. it increases with an increase in temperature
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
The correct answer is option (D): High temperature is favorable for adsorption. it increases with an increase in temperature
Was this answer helpful?
0
0
Top Questions on Van Der Waals equation
- Arrange the following gases in increasing order of van der Waals constant 'a'
A. Ar
B. CH4
C. H₂O
D. C6H6
Choose the correct option from the following.- JEE Main - 2023
- Chemistry
- Van Der Waals equation
- At low pressure, the van der Waal's equation is reduced to
- VITEEE - 2019
- Chemistry
- Van Der Waals equation
- Given van der Waals constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied ?
- NEET (UG) - 2018
- Chemistry
- Van Der Waals equation
- If $V$ is the volume of one molecule of gas under given conditions, the van der Waal?s constant $b$ is
- BITSAT - 2018
- Chemistry
- Van Der Waals equation
- The compressibility factor $(Z)$ of one mole of a van der Waals gas of negligible $�a�$ value is
- WBJEE - 2014
- Chemistry
- Van Der Waals equation
View More Questions
Questions Asked in GUJCET exam
- Which product is obtained between the reaction of CH3ONa and (CH3)3CBr?
- GUJCET - 2023
- Alcohols, Phenols and Ethers
- What is the de Broglie wavelength associated with an electron accelerated through a potential difference of 64 volts?
- GUJCET - 2023
- Refraction of Light
- Which of the following ore is not in oxide form?
- GUJCET - 2023
- General Principles and Processes of Isolation of Elements
- Which one is a reaction to prepare CCl2F2(freon-12) from CCl4?
- GUJCET - 2023
- Haloalkanes and Haloarenes
- Methylamine reacts with HNO2 to form?
View More Questions
Concepts Used:
Van Der Waals Equation
Van der Waals equation is an equation relating the relationship between the pressure, volume, temperature, and amount of real gases.
Read More: Derivation of Van Der Waals Equation
Derivation of Van der Waals equation:
For a real gas containing ‘n’ moles, the equation is written as
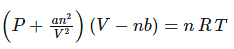
Where, P, V, T, n are the pressure, volume, temperature and moles of the gas. ‘a’ and ‘b’ constants specific to each gas.
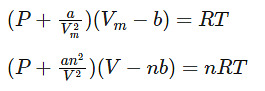
Where,
Vm: molar volume of the gas
R: universal gas constant
T: temperature
P: pressure
V: volume
Thus, Van der Waals equation can be reduced to ideal gas law as PVm = RT.
The equation can further be written as;
- Cube power of volume:
- Reduced equation (Law of corresponding states) in terms of critical constants:
Units of Van der Waals equation Constants
a: atm lit² mol-²
b: litre mol-¹



