Question:
Which of the following compounds is responsible for depletion of ozone layer?
Which of the following compounds is responsible for depletion of ozone layer?
Updated On: May 12, 2024
- Freon
- Chloroform
- D.D.T
- Iodoform
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
Freons are chlorofluorocarbons introduced into the atmosphere from aerosol sprays and refrigerating equipments. They have a very long life time and when they reach stratosphere, they undergo photochemical decomposition and destroy ozone by the following sequence of reactions :
$ {CF_2 Cl_2 ->[hv] CF_2Cl+ + Cl+ }$
$ {CFCl_3 ->[hv] CFCl_2^+ + Cl+ }$
$ { Cl^+ + O_3 -> ClO^+ +O_2}$
$ { ClO^+ + O -> Cl^+ + O_2}$
$ {CF_2 Cl_2 ->[hv] CF_2Cl+ + Cl+ }$
$ {CFCl_3 ->[hv] CFCl_2^+ + Cl+ }$
$ { Cl^+ + O_3 -> ClO^+ +O_2}$
$ { ClO^+ + O -> Cl^+ + O_2}$
Was this answer helpful?
0
0
Top Questions on Haloalkanes and Haloarenes
- For SN2 reaction, the increasing order of the reactivity of the following alkyl halides is:
(A) CH3CH2CH2CH2Br (B) CH3CH2CH(Br)CH3 (C) (CH3)3CBr (D) (CH3)2CHCH2Br- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Match List-I with List-II:

- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Although chlorine is electron-withdrawing, it is ortho- and para-directing in electrophilic substitution because • (A) Chlorine withdraws electrons through inductive effect.
(B) Chlorine destabilizes the intermediate carbocation formed during electrophilic sub-stitution.
(C) Chlorine accepts electrons through resonance
(D) Chlorine releases electrons through resonance.
Choose the correct answer from the options given below:- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- What will be the reactivity order of following compounds towards electrophilic substitution reaction ?
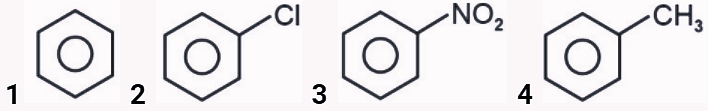
- JEE Main - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Which of the following sets of elements can be detected by Lassaigne's Test ?
- JEE Main - 2024
- Chemistry
- Haloalkanes and Haloarenes
View More Questions
Questions Asked in COMEDK UGET exam
- A $2\,kg$ mass lying on a table is displaced in the horizontal direction through $50\,cm$. The work done by the normal reaction will be
- COMEDK UGET - 2015
- work, energy and power
- The numbers of $ {H^+}$ ions present in 1 mL of a solution whose pH is 13
- COMEDK UGET - 2015
- Equilibrium
- A mixture of methylamine and dimethylamine is given to you. The reagents used to separate the components of the mixture are
- $\cot^{-1} (21) + \cot^{-1} (13) + \cot^{-1} (-8) =$
- COMEDK UGET - 2015
- Inverse Trigonometric Functions
- Pick the incorrect statement among those given below.
- COMEDK UGET - 2015
- Chemical bonding and molecular structure
View More Questions
Concepts Used:
Haloalkanes and Haloarenes
The hydrocarbons such as Haloalkanes and Haloarenes are the ones, in which one or more hydrogen atoms are replaced with halogen atoms. The main difference between Haloalkanes and Haloarenes is that Haloalkanes are derived from open chained hydrocarbons, also called alkanes, and Haloarenes are derived from aromatic hydrocarbons.
- Haloalkanes have hydrocarbons made up of aliphatic alkanes and one or more hydrogen atoms replaced by halogens (elements such as Chlorine, Bromine, Fluorine, Iodine, etc.) whereas, haloarenes consist of aromatic ring or rings and one or more hydrogen atoms replaced by halogens.
- In haloalkanes, the halogen atom is attached to the sp3 hybridized carbon atom of the alkyl group whereas, in haloarenes, the halogen atom is attached to the sp3 hybridized carbon atom of the alkyl group.
- Haloalkanes are saturated organic compounds where all the chemical bonds are attached to the carbon atom with single bonds and a single carbon atom is attached to the Halogen atom, whereas, the haloarenes differ from Haloalkanes by their method of preparation and properties.
- Haloalkanes are made by aliphatic alkanes by the process of free radical halogenation, whereas, haloarenes are made by direct halogenation of aromatic rings.
- Haloalkanes are odorless compounds, whereas, haloarenes have a sweet odor.
- Haloalkanes precipitate in SN2 substitution reactions, whereas, haloarenes do not precipitate in SN2 substitution reactions.
- Example of haloalkanes is CH3Cl (Methyl Chloride) and CH3CH2Br (Ethyl Bromide) and the example of haloarenes is Chlorobenzene, Bromobenzene.



