Question:
The reason(s) for the lower stability of $Si_2H_6$ compared to $C_2H_6$ is/are
The reason(s) for the lower stability of $Si_2H_6$ compared to $C_2H_6$ is/are
Updated On: Oct 1, 2024
- silicon is more electronegative than hydrogen
- Si-Si bond is weaker than C-C bond
- Si-H bond is weaker than C-H bond
- the presence of low-lying d-orbitals in silicon
Hide Solution
Verified By Collegedunia
The Correct Option is B, C, D
Solution and Explanation
The correct option is (B): Si-Si bond is weaker than C-C bond, (C): Si-H bond is weaker than C-H bond and (D): the presence of low-lying d-orbitals in silicon
Was this answer helpful?
0
0
Top Questions on Chemical bonding and molecular structure
- Given below are two statements :
Statement (I) : Both metal and non-metal exist in p and d-block elements.
Statement (II) : Non-metals have higher ionisation enthalpy and higher electronegativity than the metals.
In the light of the above statements, choose the most appropriate answer from the option given below:- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- Match List-I with the List-IIChoose the correct answer from the options given below:
List-I
(Compound /
Species)List-II
(Shape / Geometry)(A) \(SF_4\) (I) Tetrahedral (B) \(BrF_3\) (II) Pyramidal (C) \(BrO_{3}^{-}\) (III) See saw (D) \(NH^{+}_{4}\) (IV) Bent T-shape - JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- Number of compounds from the following with zero dipole moment is _______.
HF, H$_2$, H$_2$S, CO$_2$, NH$_3$, BF$_3$, CH$_4$, CHCl$_3$, SiF$_4$, H$_2$O, BeF$_2$- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- The fusion of chromite ore with sodium carbonate in the presence of air leads to the formation of products A and B along with the evolution of CO$_2$. The sum of spin-only magnetic moment values of A and B is ______ B.M. (Nearest integer)
(Given atomic number: C : 6, Na : 11, O : 8, Fe : 26, Cr : 24)- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
- Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): NH$_3$ and NF$_3$ molecule have pyramidal shape with a lone pair of electrons on nitrogen atom. The resultant dipole moment of NH$_3$ is greater than that of NF$_3$.
Reason (R): In NH$_3$, the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the N$-$H bonds. F is the most electronegative element.
In the light of the above statements, choose the correct answer from the options given below:- JEE Main - 2024
- Chemistry
- Chemical bonding and molecular structure
View More Questions
Questions Asked in IIT JAM CY exam
- A salt QCl of a certain metal Q is electrolyzed to its elements. 40 g of metal Q is formed at an electrode. The volume of Cl2 formed at the other electrode at 1 atm pressure and 298 K is _______ litres. (rounded off to one decimal place)
[Given: The gas constant 𝑅 = 0.082 L atm mol−1 K−1 , the molar mass of Q is 40 g mol−1 and Cl2 is assumed to be an ideal gas]- IIT JAM CY - 2024
- Electrochemistry
- Exhaustive hydrogenation of the following compound
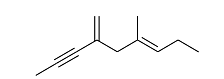
under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is _______.- IIT JAM CY - 2024
- Stereochemistry
- In the cell reaction
P+(𝑎𝑞)+Q(𝑠)→P(𝑠)+Q+(𝑎𝑞)
the EMF of the cell, 𝐸𝑐𝑒𝑙𝑙 is zero. The standard EMF of the cell, 𝐸𝑜𝑐𝑒𝑙𝑙 is
[Given:
Activities of all solids are unity.
Activity of P+(𝑎𝑞) is 2 M. Activity of Q+(𝑎𝑞) is 1 M.
𝑅 = universal gas constant; 𝑇 = temperature; 𝐹 = Faraday constant]- IIT JAM CY - 2024
- Electrochemistry
- A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
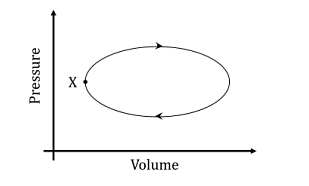
The correct statement about this process is- IIT JAM CY - 2024
- Thermodynamics
- The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:
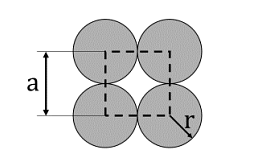
The percentage (%) area occupied by the grey circles (of radius r) inside the unit cell is _______. (rounded off to the nearest integer)- IIT JAM CY - 2024
- Solid State
View More Questions



