The number of given orbitals which have electron density along the axis is _____ $p _x, p _y, p _z, d _{x y}, d _{y z}, d _{x z}, d _z 2, d _x{ }^2-y^2$
Correct Answer: 5
Solution and Explanation
The correct answer is 5
px,py,pz,dz2 & dx2−y2 are axial orbitals.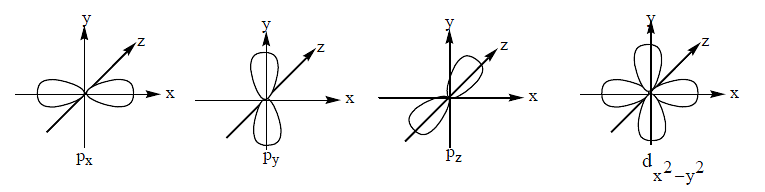

Top Questions on Molecular Orbital Theory
- Find out the maximum number of hybrid orbitals formed when \(2s\) and \(2p\) orbitals are mixed.
- JEE Main - 2024
- Chemistry
- Molecular Orbital Theory
- For $OF _2$ molecule consider the following : A Number of lone pairs on oxygen is 2 B FOF angle is less than $1045^{\circ}$ C Oxidation state of $O$ is $-2$ D Molecule is bent ' $V$ ' shaped E Molecular geometry is linear correct options are:
- JEE Main - 2023
- Chemistry
- Molecular Orbital Theory
- According to MO theory the bond orders for \(O_2^{2−},CO\,\, \text {and} \,\,NO^+\) respectively, are
- JEE Main - 2023
- Chemistry
- Molecular Orbital Theory
The correct molecular orbital diagram for F2 molecule in the ground state is
- JEE Advanced - 2023
- Chemistry
- Molecular Orbital Theory
- The number of paramagnetic species from the following is ________
$ {\left[ Ni ( CN )_4\right]^{2-} \cdot\left[ Ni ( CO )_4\right],\left[ NiCl _4\right]^{2-}} $ $ {\left[ Fe ( CN )_6\right]^{4-},\left[ Cu \left( NH _3\right)_4\right]^{2+}}$ $ {\left[ Fe ( CN )_6\right]^{3-} \text { and }\left[ Fe \left( H _2 O \right)_6\right]^{2+}}$- JEE Main - 2023
- Chemistry
- Molecular Orbital Theory
Questions Asked in JEE Main exam
- Find the acceleration of \(2\) \(kg\) block shown in the diagram (neglect friction)

- JEE Main - 2024
- Acceleration
- If \(|2A|^3 =21\) and \(\begin{bmatrix} 1 & 0 & 0 \\[0.3em] 0 & α & β \\[0.3em] 0 & β & α \end{bmatrix}\), then a is (if \(α,β∈I\))
- JEE Main - 2024
- Matrices
- Let \(α\) and \(β\) the roots of equation \(px^2 + qx - r = 0\), where \(P≠ 0\). If \(p,q,r\) be the consecutive term of non constant G.P and \(\frac{1}{α} + \frac{1}{β} = \frac{3}{4}\) then the value of \((α - β)^2\) is:
- JEE Main - 2024
- Geometric Progression
- Two lines \(L_1 \;\& \;L_2\) passing through origin trisecting the line segment intercepted by the line \(4x + 5y = 20\) between the coordinate axes. Then the tangent of angle between the lines \(L_1\) and \(L_2\) is
- JEE Main - 2024
- Tangents and Normals
- Rank of the word 'GTWENTY' in dictionary is _____ .
- JEE Main - 2024
- permutations and combinations
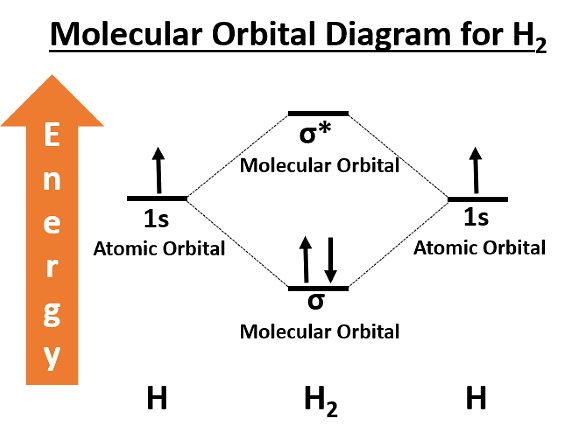
Concepts Used:
Molecular Orbital Theory
The Molecular Orbital Theory is a more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO).
Molecular Orbital theory is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Due to this arrangement in MOT Theory, electrons associated with different nuclei can be found in different atomic orbitals. In molecular orbital theory, the electrons present in a molecule are not assigned to individual chemical bonds between the atoms. Rather, they are treated as moving under the influence of the atomic nuclei in the entire molecule.