The correct molecular orbital diagram for F2 molecule in the ground state is
The correct molecular orbital diagram for F2 molecule in the ground state is
The Correct Option is C
Approach Solution - 1
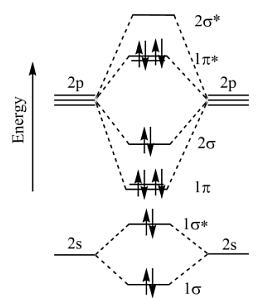
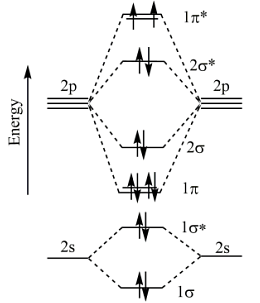
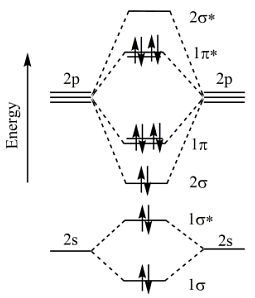
For F2 molecule (σ2s)2 (σ\(\times\)2s)2 (σ2p)2 (π2p2 = π2p2) (π\(\times\)2p2 = π\(\times\)2p2)
The provided diagram illustrates the molecular orbital configuration of the F2 molecule.
Approach Solution -2

In the ground state molecular orbital (MO) diagram for the \( \text{F}_2 \) molecule, the valence electrons of each fluorine atom participate in forming molecular orbitals. Since each fluorine atom contributes 7 valence electrons (F: 1s² 2s² 2p⁵), the total number of valence electrons in the \( \text{F}_2 \) molecule is 14.
The molecular orbital diagram for \( \text{F}_2 \) can be constructed by considering the formation and combination of atomic orbitals, followed by the filling of molecular orbitals according to the Aufbau principle, Pauli exclusion principle, and Hund's rule.
The molecular orbitals formed include:
- Bonding orbitals (\( \sigma \), \( \pi \)) which are lower in energy.
- Antibonding orbitals (\( \sigma^* \), \( \pi^* \)) which are higher in energy.
In \( \text{F}_2 \), after filling the molecular orbitals, the \( \sigma \) bonding molecular orbital is lower in energy than the \( \pi \) bonding molecular orbitals. Additionally, the \( \sigma^* \) antibonding molecular orbitals are higher in energy than their corresponding bonding orbitals. The \( \pi \) bonding molecular orbitals are higher in energy than the \( \sigma \) bonding molecular orbital but lower than the \( \sigma^* \) antibonding molecular orbitals.
This arrangement of molecular orbitals correctly represents the ground state of the \( \text{F}_2 \) molecule.
Hence The correct Answer is Option (3)
Top Questions on Molecular Orbital Theory
- Find out the maximum number of hybrid orbitals formed when \(2s\) and \(2p\) orbitals are mixed.
- JEE Main - 2024
- Chemistry
- Molecular Orbital Theory
- For $OF _2$ molecule consider the following : A Number of lone pairs on oxygen is 2 B FOF angle is less than $1045^{\circ}$ C Oxidation state of $O$ is $-2$ D Molecule is bent ' $V$ ' shaped E Molecular geometry is linear correct options are:
- JEE Main - 2023
- Chemistry
- Molecular Orbital Theory
- According to MO theory the bond orders for \(O_2^{2−},CO\,\, \text {and} \,\,NO^+\) respectively, are
- JEE Main - 2023
- Chemistry
- Molecular Orbital Theory
- The number of paramagnetic species from the following is ________
$ {\left[ Ni ( CN )_4\right]^{2-} \cdot\left[ Ni ( CO )_4\right],\left[ NiCl _4\right]^{2-}} $ $ {\left[ Fe ( CN )_6\right]^{4-},\left[ Cu \left( NH _3\right)_4\right]^{2+}}$ $ {\left[ Fe ( CN )_6\right]^{3-} \text { and }\left[ Fe \left( H _2 O \right)_6\right]^{2+}}$- JEE Main - 2023
- Chemistry
- Molecular Orbital Theory
- The number of given orbitals which have electron density along the axis is _____ $p _x, p _y, p _z, d _{x y}, d _{y z}, d _{x z}, d _z 2, d _x{ }^2-y^2$
- JEE Main - 2023
- Chemistry
- Molecular Orbital Theory
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.

Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
Concepts Used:
Molecular Orbital Theory
The Molecular Orbital Theory is a more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO).
Molecular Orbital theory is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Due to this arrangement in MOT Theory, electrons associated with different nuclei can be found in different atomic orbitals. In molecular orbital theory, the electrons present in a molecule are not assigned to individual chemical bonds between the atoms. Rather, they are treated as moving under the influence of the atomic nuclei in the entire molecule.