Name the reagents used in the following reactions:
(i)Oxidation of a primary alcohol to carboxylic acid.
(ii)Oxidation of a primary alcohol to aldehyde.
(iii)Bromination of phenol to 2,4,6-tribromophenol.
(iv)Benzyl alcohol to benzoic acid.
(v)Dehydration of propan-2-ol to propane.
(vi)Butan-2-one to butan-2-ol.
Name the reagents used in the following reactions:
(i)Oxidation of a primary alcohol to carboxylic acid.
(ii)Oxidation of a primary alcohol to aldehyde.
(iii)Bromination of phenol to 2,4,6-tribromophenol.
(iv)Benzyl alcohol to benzoic acid.
(v)Dehydration of propan-2-ol to propane.
(vi)Butan-2-one to butan-2-ol.
Solution and Explanation
(i)Acidified potassium permanganate

(ii)Pyridinium chlorochromate(PCC)
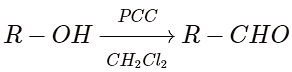
(iii) Bromine water

(iv)Acidified potassium permanganate

(v)85% phosphoric acid

(vi)NaBH4 or LiAlH4

Top Questions on Alcohols, Phenols And Ethers
- Which among the following is a trihydric alcohol?
- CUET (UG) - 2024
- Chemistry
- Alcohols, Phenols And Ethers
- Which of the following statements relating to the acidic character of alcohols is not true ?
- CUET (UG) - 2023
- Chemistry
- Alcohols, Phenols And Ethers
- In the given reaction, the end-product is-

C is-- CUET (UG) - 2023
- Chemistry
- Alcohols, Phenols And Ethers
- Which of the following compounds contain at least one secondary alcoholic group :

Choose the correct answer from the options given below :- CUET (UG) - 2023
- Chemistry
- Alcohols, Phenols And Ethers
- Cyclopropanol

Identify D- CUET (UG) - 2023
- Chemistry
- Alcohols, Phenols And Ethers
Questions Asked in CBSE CLASS XII exam
- Find the inverse of each of the matrices,if it exists \(\begin{bmatrix} 2 & 1 \\ 7 & 4 \end{bmatrix}\)
- For what values of x,\(\begin{bmatrix} 1 & 2 & 1 \end{bmatrix}\)\(\begin{bmatrix} 1 & 2 & 0\\ 2 & 0 & 1 \\1&0&2 \end{bmatrix}\)\(\begin{bmatrix} 0 \\2\\x\end{bmatrix}\)=O?
What is the Planning Process?
- CBSE CLASS XII - 2023
- Planning process steps
- Find the inverse of each of the matrices,if it exists. \(\begin{bmatrix} 2 & 3\\ 5 & 7 \end{bmatrix}\)
- Find the inverse of each of the matrices, if it exists. \(\begin{bmatrix} 1 & 3\\ 2 & 7\end{bmatrix}\)
Notes on Alcohols, Phenols And Ethers
Concepts Used:
Preparation - Alcohols, Phenols and Ethers
Alcohols, phenols, and ethers are organic compounds that can be prepared by various methods.
Preparation of Alcohols:
- Direct hydration of alkenes: Alcohols can be prepared by the addition of water to an alkene in the presence of a strong acid catalyst.
- Reduction of carbonyl compounds: Alcohols can be prepared by the reduction of aldehydes, ketones, or carboxylic acids using reducing agents like NaBH4 or LiAlH4.
- Grignard reaction: Alcohols can be prepared by reacting Grignard reagents with carbonyl compounds.
- Hydroboration-oxidation: Alcohols can be prepared by the hydroboration of alkenes followed by oxidation with an oxidizing agent like H2O2.
Preparation of Phenols:
- Hydrolysis of diazonium salts: Phenols can be prepared by the hydrolysis of diazonium salts, which are formed by the reaction of aniline with nitrous acid.
- Oxidation of sulfonic acids: Phenols can be prepared by the oxidation of sulfonic acids using strong oxidizing agents like potassium permanganate or chromic acid.
Preparation of Ethers:
- Williamson synthesis: Ethers can be prepared by the reaction of an alkoxide ion with a primary alkyl halide or tosylate in the presence of a strong base like NaOH or KOH.
- Dehydration of alcohols: Ethers can be prepared by the dehydration of alcohols in the presence of a strong acid catalyst like H2SO4.
In summary, alcohols, phenols, and ethers can be prepared by a variety of methods, including hydration, reduction, Grignard reaction, hydroboration-oxidation, hydrolysis, oxidation, Williamson synthesis, and dehydration. The choice of the method depends on the availability of starting materials, the desired product, and the conditions of the reaction.



