Question:
In which one of the following pairs, both the elements does not have (n-1)d10ns2 configuration in its elementary state?
In which one of the following pairs, both the elements does not have (n-1)d10ns2 configuration in its elementary state?
Updated On: Sep 23, 2024
- Zn,Cd
- Cd,Hg
- Hg,Cn
- Cu,Zn
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
The correct answer is (D) : Cu,Zn.
Was this answer helpful?
4
6
Top Questions on subshell electronic configuration
- Match List-I with List-II:Choose the correct answer from the options given below:
List-I ( Ions )
List-II ( No. of unpaired electrons )
A Zn$^{2+}$ (I) 0
B Cu$^{2+}$ (II) 4
C Ni$^{2+}$ (III) 1
D Fe$^{2+}$ (IV) 2
- CUET (UG) - 2024
- Chemistry
- subshell electronic configuration
- The energy of an electron in the ground state (n=1) for He+ ion is -xJ, then that for an electron in n=2 state for Be3+ ion in J is:
- NEET (UG) - 2024
- Chemistry
- subshell electronic configuration
- In which of the options, all the elements have d10 configuration in their ground state
- JEE Main - 2024
- Chemistry
- subshell electronic configuration
- The electronic configuration of Neodymium (60) Nd is
- JEE Main - 2024
- Chemistry
- subshell electronic configuration
- Which of the following compounds have colour due to d-d transition?
- JEE Main - 2024
- Chemistry
- subshell electronic configuration
View More Questions
Questions Asked in KCET exam
- A cubical Gaussian surface has side of length a = 10 cm. Electric field lines are parallel to x-axis as shown. The magnitudes of electric fields through surfaces ABCD and EFGH are 6kNC-1 and 9kNC-1 respectively. Then the total charge enclosed by the cube is
[Take ε0 = 9 × 10-12 Fm-1]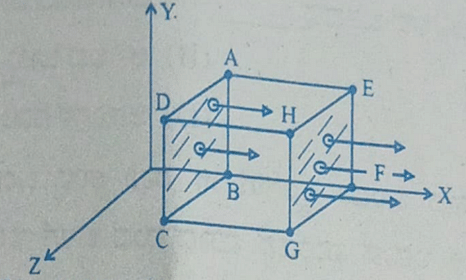
- KCET - 2023
- Gauss Law
- A sample of water is found to contain 5.85% \((\frac{w}{w})\) of AB (molecular mass 58.5) and 9.50% \((\frac{w}{w})\) XY2 (molecular mass 95). Assuming 80% ionisation of AB and 60% ionisation of XY2 , the freezing point of water sample is
[Given : Kf for water 1.86 K kg mol-1 , Freezing point of pure water is 273 K and A, B and Y are monovalent ions]- KCET - 2023
- Colligative Properties
- \(aMnO^{-}_4+bS_2O_{3}^-+H_2O\rightarrow xMnO_2+ySO_{4}^-+zOH^-\)
a and y respectively are- KCET - 2023
- Redox reactions
- \(\int\sqrt{\cosec x-\sin x}\ dx=\)
- KCET - 2023
- Definite Integral
- A metallic rod of length 1 m held along east-west direction is allowed to fall down freely. Given horizontal component of earth’s magnetic field BH = 3 × 10-5 T. The emf induced in the rod at an instant t = 2s after it is released is ( Take g = 10 ms-2 )
- KCET - 2023
- Faradays laws of induction
View More Questions



