Question:
A sample of water is found to contain 5.85% \((\frac{w}{w})\) of AB (molecular mass 58.5) and 9.50% \((\frac{w}{w})\) XY2 (molecular mass 95). Assuming 80% ionisation of AB and 60% ionisation of XY2 , the freezing point of water sample is
[Given : Kf for water 1.86 K kg mol-1 , Freezing point of pure water is 273 K and A, B and Y are monovalent ions]
A sample of water is found to contain 5.85% \((\frac{w}{w})\) of AB (molecular mass 58.5) and 9.50% \((\frac{w}{w})\) XY2 (molecular mass 95). Assuming 80% ionisation of AB and 60% ionisation of XY2 , the freezing point of water sample is
[Given : Kf for water 1.86 K kg mol-1 , Freezing point of pure water is 273 K and A, B and Y are monovalent ions]
[Given : Kf for water 1.86 K kg mol-1 , Freezing point of pure water is 273 K and A, B and Y are monovalent ions]
Updated On: Nov 7, 2024
- 264.25 K
- 265.56 K
- 280.44 K
- 281.75 K
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
The correct answer is (A) : 264.25 K.
Was this answer helpful?
12
11
Top Questions on Colligative Properties
- The bloom strength is directly proportional to:
- GPAT - 2024
- Physical Chemistry
- Colligative Properties
- Lead storage battery contains $38 \%$ by weight solution of $H _2 SO _4$ The van't Hoff factor is $2.67$ at this concentration The temperature in Kelvin at which the solution in the battery will freeze is ______(Nearest integer) Given $K _f=1.8\, K \,kg\, mol ^{-1}$
- JEE Main - 2023
- Chemistry
- Colligative Properties
- Match List I and List IIChoose the correct answer from the options given below :
List II List II A. van't Hoff factor, i I. Cryoscopic constant B. $k_f$ II. Isotonic solutions C. Solution with same with same osmotic pressure III. Normal molar mass /Abnormal molar mass D. Azeotropes IV. Solutions with same composition of vapour above it - JEE Main - 2023
- Chemistry
- Colligative Properties
- Two solutions $A$ and $B$ are prepared by dissolving $1 g$ of non-volatile solutes $X$ and $Y$ respectively in $1 \,kg$ of water The ratio of depression in freezing points for $A$ and $B$ is found to be $1: 4$ The ratio of molar masses of $X$ and $Y$ is :
- JEE Main - 2023
- Chemistry
- Colligative Properties
- Evaluate the following statements for their correctness
A. The elevation in boiling point temperature of water will be same for $01 \,M \,NaCl$ and $01\, M$ urea
B. Azeotropic mixtures boil without change in their composition
C. Osmosis always takes place from hypertonic to hypotonic solution
D. The density of $32 \% H _2 SO _4$ solution having molarity $409\, M$ is approximately $126\, g mL ^{-1}$
E. A negatively charged sol is obtained when KI solution is added to silver nitrate solution
Choose the correct answer from the options given below :- JEE Main - 2023
- Chemistry
- Colligative Properties
View More Questions
Questions Asked in KCET exam
- A cubical Gaussian surface has side of length a = 10 cm. Electric field lines are parallel to x-axis as shown. The magnitudes of electric fields through surfaces ABCD and EFGH are 6kNC-1 and 9kNC-1 respectively. Then the total charge enclosed by the cube is
[Take ε0 = 9 × 10-12 Fm-1]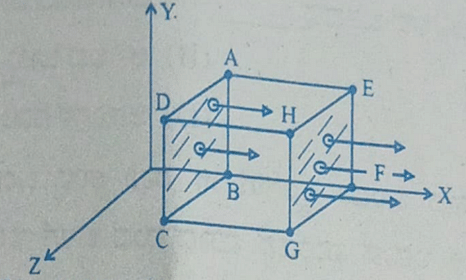
- KCET - 2023
- Gauss Law
- \(aMnO^{-}_4+bS_2O_{3}^-+H_2O\rightarrow xMnO_2+ySO_{4}^-+zOH^-\)
a and y respectively are- KCET - 2023
- Redox reactions
- \(\int\sqrt{\cosec x-\sin x}\ dx=\)
- KCET - 2023
- Definite Integral
- A metallic rod of length 1 m held along east-west direction is allowed to fall down freely. Given horizontal component of earth’s magnetic field BH = 3 × 10-5 T. The emf induced in the rod at an instant t = 2s after it is released is ( Take g = 10 ms-2 )
- KCET - 2023
- Faradays laws of induction
- The P-V diagram of a Carnot's engine is shown in the graph below. The engine uses 1 mole of an ideal gas as working substance. From the graph, the area enclosed by the P-V diagram is
[ The heat supplied to the gas is 8000 J ]
- KCET - 2023
- carnot cycle
View More Questions



