In a one-litre flask, 6 moles of A undergoes the reaction A (g) ⇌ P (g). The progress of product formation at two temperatures (in Kelvin), T1 and T2, is shown in the figure:

If T1 = 2T2 and (∆G2Θ − ∆G1Θ) = RT2 ln x, then the value of x is ___ .
[∆G1Θ and ∆G2Θ are standard Gibb’s free energy change for the reaction at temperatures T1 and T2, respectively.]

If T1 = 2T2 and (∆G2Θ − ∆G1Θ) = RT2 ln x, then the value of x is ___ .
[∆G1Θ and ∆G2Θ are standard Gibb’s free energy change for the reaction at temperatures T1 and T2, respectively.]
Correct Answer: 8
Solution and Explanation
The answer is 8.
Top Questions on Law Of Chemical Equilibrium And Equilibrium Constant
- When potassium iodide is added to an aqueous solution of potassium ferricyanide, a reversible reaction is observed in which a complex P is formed. In a strong acidic medium, the equilibrium shifts completely towards P. Addition of zinc chloride to P in a slightly acidic medium results in a sparingly soluble complex Q.
- JEE Advanced - 2024
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Which one of the following has the same number of atoms as are in 6g of H2O
- TS EAMCET - 2023
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- At 500 K, for a reversible reaction \(A_{2_{(g)}}+B_{2_{(g)}}⇌2AB_{(g)}\) in a closed container, KC = 2 × 10-5. In the presence of catalyst, the equilibrium is attaining 10 times faster. The equilibrium constant KC in the presence of catalyst at the same temperature is
- KCET - 2023
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Which of the following does not form a buffer solution?
- TS EAMCET - 2023
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- (i) $ X ( g ) \rightleftharpoons Y ( g )+ Z$ (g) $ K _{ p 1}=3$ (ii) $A ( g ) \rightleftharpoons 2 B ( g )\,\, K _{ p 2}=1$ If the degree of dissociation and initial concentration of both the reactants $X ( g )$ and $A ( g )$ are equal, then the ratio of the total pressure at equilibrium $\left(\frac{p_1}{p_2}\right)$ is equal to $x : 1$ The value of $x$ is _________ (Nearest integer)
- JEE Main - 2023
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
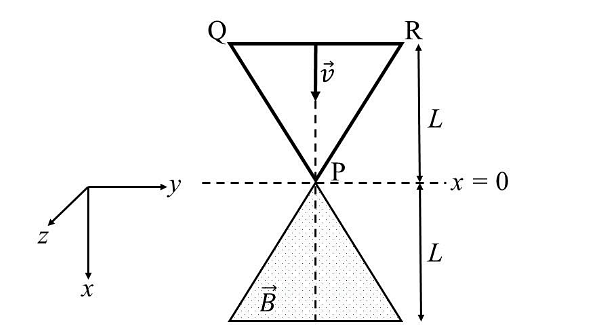
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
Concepts Used:
Law of Chemical Equilibrium
Law of Chemical Equilibrium states that at a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation.
Let us consider a general reversible reaction;
A+B ↔ C+D
After some time, there is a reduction in reactants A and B and an accumulation of the products C and D. As a result, the rate of the forward reaction decreases and that of backward reaction increases.
Eventually, the two reactions occur at the same rate and a state of equilibrium is attained.
By applying the Law of Mass Action;
The rate of forward reaction;
Rf = Kf [A]a [B]b
The rate of backward reaction;
Rb = Kb [C]c [D]d
Where,
[A], [B], [C] and [D] are the concentrations of A, B, C and D at equilibrium respectively.
a, b, c, and d are the stoichiometric coefficients of A, B, C and D respectively.
Kf and Kb are the rate constants of forward and backward reactions.
However, at equilibrium,
Rate of forward reaction = Rate of backward reaction.
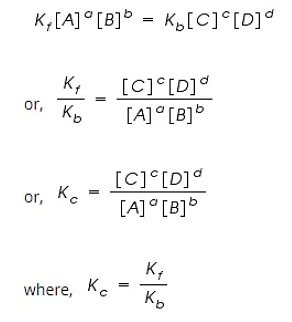
Kc is called the equilibrium constant expressed in terms of molar concentrations.
The above equation is known as the equation of Law of Chemical Equilibrium.



