For the reaction, $X (s) \rightleftharpoons Y (s)+ Z (g)$, the plot of $\ln \frac{p_{ Z }}{p^{0}}$ versus $\frac{10^{4}}{T}$ is given below (in solid line), where $p_{ z }$ is the pressure (in bar) of the gas $Z$ at temperature $T$ and $p^0=1$ bar
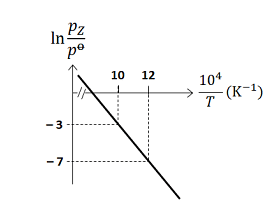
(Given, $\frac{ d (\ln K)}{ d \left(\frac{1}{T}\right)}=-\frac{\Delta H^{0}}{R}$, where the equilibrium constant, $K=\frac{p_{z}}{p^{0}}$ and the gas constant, $R=8.314 \,J \,K ^{-1} mol ^{-1}$ )
The value of $\Delta S^{0}$ (in $J\, K ^{-1} mol ^{-1}$ ) for the given reaction, at $1000\, K$ is ______