An ideal diatomic gas is made up of molecules that do not vibrate. Its volume compressed by a factor of 32,without any exchange of heat. If the initial and final pressures are P1 and P2,respectively,the ratio P1:P2,is:
An ideal diatomic gas is made up of molecules that do not vibrate. Its volume compressed by a factor of 32,without any exchange of heat. If the initial and final pressures are P1 and P2,respectively,the ratio P1:P2,is:
7:5
128:1
1:32
32:1
3:08
The Correct Option is B
Approach Solution - 1
The correct answer is (B): \(128:1\)
Approach Solution -2
To determine the ratio of the initial pressure \(P_1\) to the final pressure \(P_2\) for an ideal diatomic gas (which does not vibrate) undergoing adiabatic compression, we need to use the adiabatic condition for an ideal gas.
For an ideal diatomic gas, which has 5 degrees of freedom (3 translational + 2 rotational), the adiabatic index (γ) is:
\[ \gamma = \frac{7}{5} = 1.4 \]
The adiabatic relation for pressure and volume is given by:
\[ P_1 V_1^\gamma = P_2 V_2^\gamma \]
Here, \( V_1 \) is the initial volume and \( V_2 \) is the final volume. The problem states that the volume is compressed by a factor of 32, so:
\[ V_2 = \frac{V_1}{32} \]
Using the adiabatic condition, we have:
\[ P_1 V_1^\gamma = P_2 V_2^\gamma \]
\[ P_1 V_1^\gamma = P_2 \left( \frac{V_1}{32} \right)^\gamma \]
\[ P_1 V_1^\gamma = P_2 \frac{V_1^\gamma}{32^\gamma} \]
\[ P_1 = P_2 \frac{1}{32^\gamma} \]
\[ \frac{P_1}{P_2} = 32^\gamma \]
Substitute \(\gamma = 1.4\) into the equation:
\[ \frac{P_1}{P_2} = 32^{1.4} \]
Now we calculate \( 32^{1.4} \):
\[ 32 = 2^5 \]
\[ 32^{1.4} = (2^5)^{1.4} = 2^{5 \cdot 1.4} = 2^7 = 128 \]
Therefore, the ratio \( P_1 : P_2 \) is:
\[ P_1 : P_2 = 128 \]
So, the final answer is:
\[ P_1 : P_2 = 128 : 1 \]
Thus The correct answer is Option (B): \(128:1\)
Top Questions on Thermodynamics
- Which of the following is correct for adiabatic free expansion against vacuum?
- JEE Main - 2024
- Chemistry
- Thermodynamics
- In an air-standard Otto cycle, the pressure and temperature of air just before the compression stroke are 200 kPa and 26.85 °C, respectively. The combustion process is assumed to be a constant volume process, where 1.02 MJ/kg heat is added. The cycle efficiency is 50%. The adiabatic index \(γ\) and specific heat at constant volume \(C_v\) can be considered to be constant during the process (corresponding values taken at the mean cycle temperature).
Assuming that the ideal gas law is applicable, \(γ = \frac 43\) and \(c_v= 0.85\ kJ/kg-K\), the maximum pressure (in MPa) reached during the cycle is _____ . (Rounded off to 1 decimal place)- GATE PI - 2024
- General Engineering
- Thermodynamics
- Match List I with List II
List-I
(Process)List-II
(Conditions)(A) Isothermal process (I) No heat exchange (B) Isochoric process (II) Carried out at constant temperature (C) Isobaric process (III) Carried out at constant volume (D) Adiabatic process (IV) Carried out at constant pressure
Choose the correct answer from the options given below:- NEET (UG) - 2024
- Chemistry
- Thermodynamics
- Consider the two statements (Assume density of water to be constant):
Statement 1: A capillary tube is first dipped in hot water and then dipped in cold water. The rise is higher in hot water.
Statement 2: Capillary tube is first dipped in cold water and then in hot water. The rise is higher in cold water.- JEE Main - 2024
- Physics
- Thermodynamics
- The Pressure (P) versus volume (V) of thermodynamic process shown in figure. The select the correct options (Take γ= 1.1)
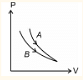
- JEE Main - 2024
- Physics
- Thermodynamics
Questions Asked in KEAM exam
- If the two sides AB and AC of a triangle are along \(4x-3y-17 = 0\) and \(3x+4y-19= 0\), then the equation of the bisector of the angle between AB and AC is ?
- KEAM - 2023
- Straight lines
- An average frictional force of 80N is required to stop an object at a distance of 25m. If the initial speed of the object is 20m/s,the mass of the object is:
- KEAM - 2023
- work, energy and power
- A biased die is rolled such that the probability of getting k dots,1≤k≤6, on the upper face of the die is proportional to k. Then the probability that five dots appear on the upper face of the die is
- KEAM - 2023
- Probability
- A planet has an escape speed of 10km/s,The radius of the planet is 10,000km. The acceleration due to gravity of the planet at its surface is:
- KEAM - 2023
- Gravitation
- \(∫xlog(1+x^2)dx=\)?
- KEAM - 2023
- Integration by Parts
Concepts Used:
Thermodynamics
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.
Important Terms
System
A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. The system boundary may be real or imaginary, fixed or deformable.
There are three types of systems:
- Isolated System – An isolated system cannot exchange both energy and mass with its surroundings. The universe is considered an isolated system.
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place. Refrigerators and compression of gas in the piston-cylinder assembly are examples of closed systems.
- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
Thermodynamic Process
A system undergoes a thermodynamic process when there is some energetic change within the system that is associated with changes in pressure, volume and internal energy.
There are four types of thermodynamic process that have their unique properties, and they are:
- Adiabatic Process – A process in which no heat transfer takes place.
- Isochoric Process – A thermodynamic process taking place at constant volume is known as the isochoric process.
- Isobaric Process – A process in which no change in pressure occurs.
- Isothermal Process – A process in which no change in temperature occurs.
Laws of Thermodynamics
Zeroth Law of Thermodynamics
The Zeroth law of thermodynamics states that if two bodies are individually in equilibrium with a separate third body, then the first two bodies are also in thermal equilibrium with each other.
First Law of Thermodynamics
The First law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing three kinds of transfer of energy, as heat, as thermodynamic work, and as energy associated with matter transfer, and relating them to a function of a body's state, called internal energy.
Second Law of Thermodynamics
The Second law of thermodynamics is a physical law of thermodynamics about heat and loss in its conversion.
Third Law of Thermodynamics
Third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: The entropy of a system approaches a constant value when its temperature approaches absolute zero.



