Question:
A pure compound contains 2.4g of C, 1.2 × 1023 atoms of H, 0.2 moles of oxygen atoms. Its empirical formula is
A pure compound contains 2.4g of C, 1.2 × 1023 atoms of H, 0.2 moles of oxygen atoms. Its empirical formula is
Updated On: Oct 8, 2024
- C2HO
- C2H2O2
- CH2O
- CHO
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
The correct answer is (D) : CHO.
Was this answer helpful?
2
4
Top Questions on Stoichiometry and Stoichiometric Calculations
- 9.3 g of pure aniline is treated with bromine water at room temperature to give a white precipitate of the product 'P'. The mass of product 'P' obtained is 26.4 g. The percentage yield is ......... %.
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- \( X \, \text{g} \) of ethylamine is subjected to reaction with \( \text{NaNO}_2/\text{HCl} \) followed by water; evolved dinitrogen gas which occupied \( 2.24 \, \text{L} \) volume at STP. \( X \) is ______ \( \times 10^{-1} \, \text{g} \).
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- If 50 mL of 0.5 M oxalic acid is required to neutralise 25 mL of NaOH solution, the amount of NaOH in 50 mL of given NaOH solution is _____g.
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- Consider the following reaction:\[3\text{PbCl}_2 + 2(\text{NH}_4)_3\text{PO}_4 \rightarrow \text{Pb}_3(\text{PO}_4)_2 + 6\text{NH}_4\text{Cl}\]If 72 mmol of PbCl\(_2\) is mixed with 50 mmol of (\(\text{NH}_4\))\(_3\)PO\(_4\), then the amount of \(\text{Pb}_3(\text{PO}_4)_2\) formed is ______ mmol (nearest integer).
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- The strength of $50$ volume solution of hydrogen peroxide is _____$g / L$ (Nearest integer)
Given: Molar mass of $H _2 O _2$ is $34\, g \, mol ^{-1}$ Molar volume of gas at $STP =22.7\, L$- JEE Main - 2023
- Chemistry
- Stoichiometry and Stoichiometric Calculations
View More Questions
Questions Asked in KCET exam
- The P-V diagram of a Carnot's engine is shown in the graph below. The engine uses 1 mole of an ideal gas as working substance. From the graph, the area enclosed by the P-V diagram is
[ The heat supplied to the gas is 8000 J ]
- KCET - 2023
- carnot cycle
- A point object is moving at a constant speed of 1 ms-1 along the principal axis of a convex lens of focal length 10cm. The speed of the image is also 1 ms-1 , when the object is at _______ cm from the optic centre of the lens.
- KCET - 2023
- spherical lenses
- A cubical Gaussian surface has side of length a = 10 cm. Electric field lines are parallel to x-axis as shown. The magnitudes of electric fields through surfaces ABCD and EFGH are 6kNC-1 and 9kNC-1 respectively. Then the total charge enclosed by the cube is
[Take ε0 = 9 × 10-12 Fm-1]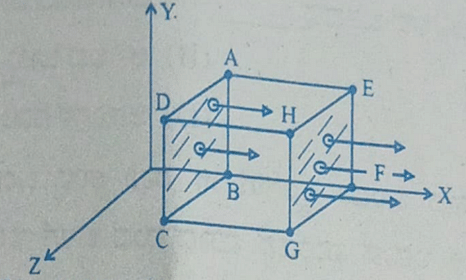
- KCET - 2023
- Gauss Law
- The modulus of the complex number \(\frac{(1+i)^2(1+3i)}{(2-6i)(2-2i)}\) is
- KCET - 2023
- complex numbers
- The value of \(\begin{vmatrix} \sin^2 14 \degree & \sin^2 66\degree & \tan 135\degree \\ \sin^2 66\degree & \tan 135\degree & \sin^2 14 \degree \\ \tan 135\degree & \sin^2 14 \degree & \sin^2 66\degree \end{vmatrix}\)
- KCET - 2023
- Trigonometric Identities
View More Questions



