While charging the lead storage battery
Show Hint
During the charging process of a lead storage battery, specific reactions occur at the cathode and anode, resulting in the reduction and oxidation of lead sulfate compounds.
- $PbSO_4$ on anode is reduced to $Pb$
- $PbSO_4$ on cathode is reduced to $Pb$
- $PbSO_4$ on cathode is oxidized to $Pb$
- $PbSO_4$ on anode is oxidized to $PbO_2$
The Correct Option is D
Approach Solution - 1
The cell reactions during charging of lead storage battery are
At anode
\(PbSO_{4}\left(s\right)+ 2e^{-} \to Pb\left(s\right)+ SO^{2-}_{4}\left(aq\right)\)
At cathode
\(PbSO_{4}\left(s\right)+ 2H_{2}O\left(l\right) \to PbO_{2}\left(s\right)+ SO^{2-}_{4}\left(aq\right) + 4H^{+} \left(aq\right)+2e^{-}\)
So, \(PbSO_4\) at the anode is reduced to \(Pb\).
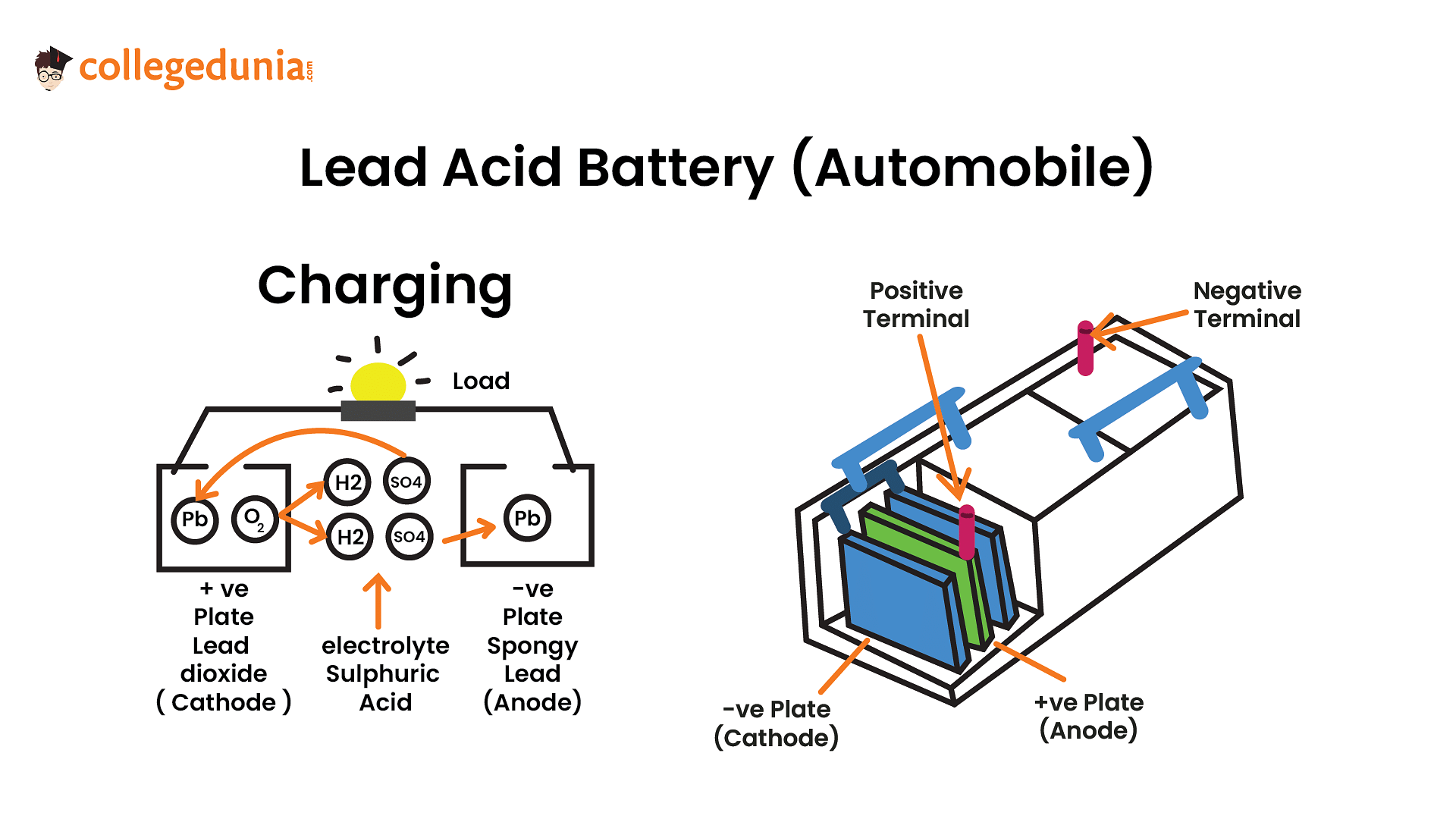
During charging, PbO2 is formed at the anode from PbSO4 (oxidation of Pb ion) and Pb is formed at the cathode from PbSO4 (reduction of Pb ion).
Therefore Options B and D are the correct answers.
Discover More From Chapter: Electrochemistry
Approach Solution -2
The Correct Answer is (D): \(PbSO_4\) on anode is oxidized to \(PbO_2\)
Real Life Applications
Applications of the Charging Process of Lead Storage Battery:
1. Automotive Power: Lead storage batteries power cars, facilitating engine ignition and electrical system operation. Charging reverses lead sulfate crystal growth on battery plates, restoring its capacity.
2. Backup Generators: Lead storage batteries serve as backup power sources for generators during outages. Charging the battery ensures it can supply essential appliances and equipment when needed.
3. Uninterrupted Power Supply (UPS): UPS systems utilize lead storage batteries to provide backup power to sensitive equipment like computers. Charging enables the battery to support devices until regular power is restored. By using the charging process to reverse chemical reactions, lead storage batteries remain reliable power sources across various applications.
Question can also be asked as
- What happens at the anode and cathode during the charging of a lead storage battery?
- How are lead sulfate (PbSO4) and lead dioxide (PbO2) formed during the charging of a lead storage battery?
- What are the oxidation and reduction reactions that occur during the charging of a lead storage battery?
Approach Solution -3
The Correct Answer is (D): \(PbSO_4\) on anode is oxidized to \(PbO_2\)
The charging process of a lead storage battery involves a series of chemical reactions that convert lead sulfate compounds back to their original forms.
- The cathode of a lead storage battery is a crucial component involved in the charging process.
- During charging, lead sulfate (PbSO4) on the cathode undergoes reduction, leading to the formation of metallic lead (Pb).
- This reduction reaction helps restore the cathode to its original state and allows the battery to store electrical energy.
- The anode of a lead storage battery plays an equally important role in the charging process.
- At the anode, lead sulfate (PbSO4) is oxidized, resulting in the formation of lead dioxide (PbO2).
- This oxidation reaction allows the anode to recover its original composition and prepares the battery for subsequent discharge.
The charging process of a lead storage battery involves the simultaneous reduction of lead sulfate at the cathode and oxidation of lead sulfate at the anode. Through these redox reactions, the battery replenishes its energy storage capacity, enabling it to deliver electrical power when needed.
Also Check:
Learn with videos:
Top Questions on Electrochemistry
- The amount of electricity in Coulomb required for the oxidation of 1 mol of \( \text{H}_2\text{O} \) to \( \text{O}_2 \) is \[\_ \times 10^5 \, \text{C}.\]
- JEE Main - 2024
- Chemistry
- Electrochemistry
- The strongest reducing agent amont the following is:
- JEE Main - 2024
- Chemistry
- Electrochemistry
- A conductivity cell with two electrodes (dark side) are half filled with infinitely dilute aqueous solution of a weak electrolyte. If volume is doubled by adding more water at constant temperature, the molar conductivity of the cell will -

- JEE Main - 2024
- Chemistry
- Electrochemistry
- How can an electrochemical cell be converted into an electrolytic cell ?
- JEE Main - 2024
- Chemistry
- Electrochemistry
- For the electrochemical cell
M|M$^{2+}$||X$^{2-}$|X
If $E^0_{(M^{2+}/M)} = 0.46$ V and $E^0_{(X/X^{2-})} = 0.34$ V.
Which of the following is correct?- JEE Main - 2024
- Chemistry
- Electrochemistry
Questions Asked in KCET exam
- If \(\lim\limits_{x \rightarrow 0} \frac{\sin(2+x)-\sin(2-x)}{x}\)= A cos B, then the values of A and B respectively are
- KCET - 2023
- limits of trigonometric functions
- The Curie temperatures of Cobalt and iron are 1400K and 1000K respectively. At T = 1600K , the ratio of magnetic susceptibility of Cobalt to that of iron is
- KCET - 2023
- Magnetism and matter
- A particle is in uniform circular motion. Related to one complete revolution of the particle, which among the stataments is incorrect ?
- KCET - 2023
- Uniform Circular Motion
- The modulus of the complex number \(\frac{(1+i)^2(1+3i)}{(2-6i)(2-2i)}\) is
- KCET - 2023
- complex numbers
- The energy gap of an LED is 2.4 eV. When the LED is switched ‘ON’, the momentum of the emitted photons is
- KCET - 2023
- Semiconductor electronics: materials, devices and simple circuits
Concepts Used:
Electrochemical Cells
An electrochemical cell is a device that is used to create electrical energy through the chemical reactions which are involved in it. The electrical energy supplied to electrochemical cells is used to smooth the chemical reactions. In the electrochemical cell, the involved devices have the ability to convert the chemical energy to electrical energy or vice-versa.
Classification of Electrochemical Cell:
Cathode
- Denoted by a positive sign since electrons are consumed here
- A reduction reaction occurs in the cathode of an electrochemical cell
- Electrons move into the cathode
Anode
- Denoted by a negative sign since electrons are liberated here
- An oxidation reaction occurs here
- Electrons move out of the anode
Types of Electrochemical Cells:
Galvanic cells (also known as Voltaic cells)
- Chemical energy is transformed into electrical energy.
- The redox reactions are spontaneous in nature.
- The anode is negatively charged and the cathode is positively charged.
- The electrons originate from the species that undergo oxidation.
Electrolytic cells
- Electrical energy is transformed into chemical energy.
- The redox reactions are non-spontaneous.
- These cells are positively charged anode and negatively charged cathode.
- Electrons originate from an external source.




