Question:
Which one of the following is correct for all elements from $Sc$ to $Cu$ ?
Which one of the following is correct for all elements from $Sc$ to $Cu$ ?
Updated On: Jul 6, 2024
- The lowest oxidation state shown by them is +2
- 4 S orbital is completely filled in the ground state
- 3 d orbital is not completely filled in the ground state
- The ions in +2 oxidation states are paramagnetic
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
Paramagnetism is due to the presence of one or more unpaired electrons
Was this answer helpful?
3
0
Top Questions on d block elements
- The d-d transitions in [Mn(H2O)6]2+ and [Ti(H2O)4]3+ , respectively, are [Ignore spin-orbit coupling and Jahn-Teller distortion.]
- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- The correct statement(s) about the ligand substitution/exchange reaction is/are
- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 are examples of
- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- Among [Ti(H2O)6]3+,[NiCl4]2−,[CrO4]2−,and [Mn(H2O)6]2+, the complex that exhibits the largest molar absorptivity in the visible region of the electronic absorption spectrum is
- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- The number and nature of d−d transition(s) in the case of Sc2+ in an octahedral crystal field, respectively, are
[Ignore spin-orbit coupling and Jahn-Teller distortion.]- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
View More Questions
Questions Asked in KCET exam
- The modulus of the complex number \(\frac{(1+i)^2(1+3i)}{(2-6i)(2-2i)}\) is
- KCET - 2023
- complex numbers
- The P-V diagram of a Carnot's engine is shown in the graph below. The engine uses 1 mole of an ideal gas as working substance. From the graph, the area enclosed by the P-V diagram is
[ The heat supplied to the gas is 8000 J ]
- KCET - 2023
- carnot cycle
- A point object is moving at a constant speed of 1 ms-1 along the principal axis of a convex lens of focal length 10cm. The speed of the image is also 1 ms-1 , when the object is at _______ cm from the optic centre of the lens.
- KCET - 2023
- spherical lenses
- A cubical Gaussian surface has side of length a = 10 cm. Electric field lines are parallel to x-axis as shown. The magnitudes of electric fields through surfaces ABCD and EFGH are 6kNC-1 and 9kNC-1 respectively. Then the total charge enclosed by the cube is
[Take ε0 = 9 × 10-12 Fm-1]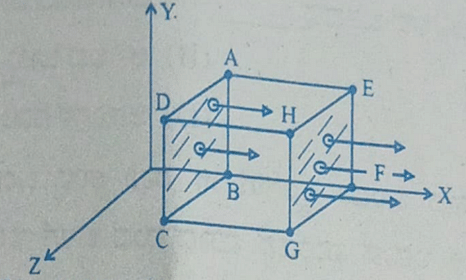
- KCET - 2023
- Gauss Law
- The value of \(\begin{vmatrix} \sin^2 14 \degree & \sin^2 66\degree & \tan 135\degree \\ \sin^2 66\degree & \tan 135\degree & \sin^2 14 \degree \\ \tan 135\degree & \sin^2 14 \degree & \sin^2 66\degree \end{vmatrix}\)
- KCET - 2023
- Trigonometric Identities
View More Questions
Concepts Used:
d block elements
The elements, in the columns of the periodic table in which d subshells are being occupied are known as d block elements.
These are the elements that have the capability of forming stable cations with incompletely filled d orbitals. Elements like mercury and Zinc are not considered transition metals because they have electronic configurations: (n-1)d10 ns2. These elements have filled d-orbitals in their ground state and, therefore, even in some of their oxidation states.
General Properties Of d-Block Elements
- Multiple oxidation states- The oxidation states of d block elements show very few energy gaps; therefore, they exhibit many oxidation states. Also, the energy difference between s and d orbital is very less. Therefore both the electrons are involved in ionic and covalent bond formation, which ultimately leads to multiple oxidation states.
- Formation of complex compounds- Ligands show a binding behaviour and can form so many stable complexes with the help of transition metals. This property is mainly due to:
- Availability of vacant d orbitals.
- Comparatively small sizes of metals.
- Hardness- Transition elements are tough and have high densities because of the presence of unpaired electrons.
- Melting and boiling points- Melting and boiling points of transition are very high. This is because of the presence of unpaired electrons and partially filled d orbitals. Because of these two things, they form strong bonds and therefore have high melting and boiling points.
- Atomic radii- The atomic and ionic radius of the transition elements decreases as we move from Group 3 to group 6. However, it remains the same between group 7 and group 10, and from group 11 to group 12 increases.
- Ionization enthalpy- The ionization enthalpies of the transition elements are generally on the greater side as compared to the S block elements



