Question:
Among [Ti(H2O)6]3+,[NiCl4]2−,[CrO4]2−,and [Mn(H2O)6]2+, the complex that exhibits the largest molar absorptivity in the visible region of the electronic absorption spectrum is
Among [Ti(H2O)6]3+,[NiCl4]2−,[CrO4]2−,and [Mn(H2O)6]2+, the complex that exhibits the largest molar absorptivity in the visible region of the electronic absorption spectrum is
Updated On: Oct 1, 2024
- [Ti(H2O)6]3+
- [NiCl4]2−
- [CrO4]2−
- [Mn(H2O)6]2+
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
The correct option is (C): [CrO4]2−
Was this answer helpful?
0
0
Top Questions on d block elements
- The correct statement(s) about the ligand substitution/exchange reaction is/are
- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- For the reaction shown below
![1/2 Mn2 (CO)10+Na Na[Mn(CO)5 ] CH3Cl-NaCl [CH3Mn(CO)5](https://images.collegedunia.com/public/qa/images/content/2024_07_31/image_a08f12a11722411541392.png)
the oxidation states of Mn in P and Q, respectively, are- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- [Co(NH3)5(SO4)]Br and [Co(NH3)5Br]SO4 are examples of
- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- The d-d transitions in [Mn(H2O)6]2+ and [Ti(H2O)4]3+ , respectively, are [Ignore spin-orbit coupling and Jahn-Teller distortion.]
- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
- The number and nature of d−d transition(s) in the case of Sc2+ in an octahedral crystal field, respectively, are
[Ignore spin-orbit coupling and Jahn-Teller distortion.]- IIT JAM CY - 2024
- Inorganic Chemistry
- d block elements
View More Questions
Questions Asked in IIT JAM CY exam
- In the cell reaction
P+(𝑎𝑞)+Q(𝑠)→P(𝑠)+Q+(𝑎𝑞)
the EMF of the cell, 𝐸𝑐𝑒𝑙𝑙 is zero. The standard EMF of the cell, 𝐸𝑜𝑐𝑒𝑙𝑙 is
[Given:
Activities of all solids are unity.
Activity of P+(𝑎𝑞) is 2 M. Activity of Q+(𝑎𝑞) is 1 M.
𝑅 = universal gas constant; 𝑇 = temperature; 𝐹 = Faraday constant]- IIT JAM CY - 2024
- Electrochemistry
- A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
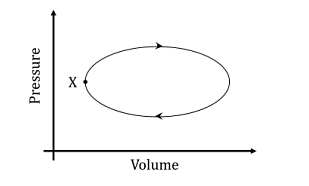
The correct statement about this process is- IIT JAM CY - 2024
- Thermodynamics
- A salt QCl of a certain metal Q is electrolyzed to its elements. 40 g of metal Q is formed at an electrode. The volume of Cl2 formed at the other electrode at 1 atm pressure and 298 K is _______ litres. (rounded off to one decimal place)
[Given: The gas constant 𝑅 = 0.082 L atm mol−1 K−1 , the molar mass of Q is 40 g mol−1 and Cl2 is assumed to be an ideal gas]- IIT JAM CY - 2024
- Electrochemistry
- The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:
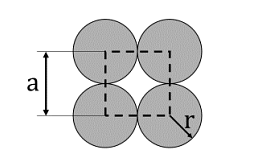
The percentage (%) area occupied by the grey circles (of radius r) inside the unit cell is _______. (rounded off to the nearest integer)- IIT JAM CY - 2024
- Solid State
- The ratio of osmotic pressures of aqueous solutions of 0.01 M BaCl2 to 0.005 M NaCl is
[Given: Both compounds dissociate completely in water]- IIT JAM CY - 2024
- Chemical equilibria
View More Questions



