Which one among the following metals is the weakest reducing agent?
- $Rb$
- $Na$
- $Li$
- $K$
The Correct Option is B
Approach Solution - 1
Sodium has the lowest oxidation potential in alkali metals. Hence it is the weakest reducing agent among alkali metals. Low ionization energy should be present for the element to act as a reducing agent. Alkali metals have low ionization energy thus making them a strong reducing agent.
So, the correct option is (B): Na.
Read more from the chapter: Classification of Elements and Periodicity in Properties
Approach Solution -2
The Correct Answer is (B)
Real Life Applications
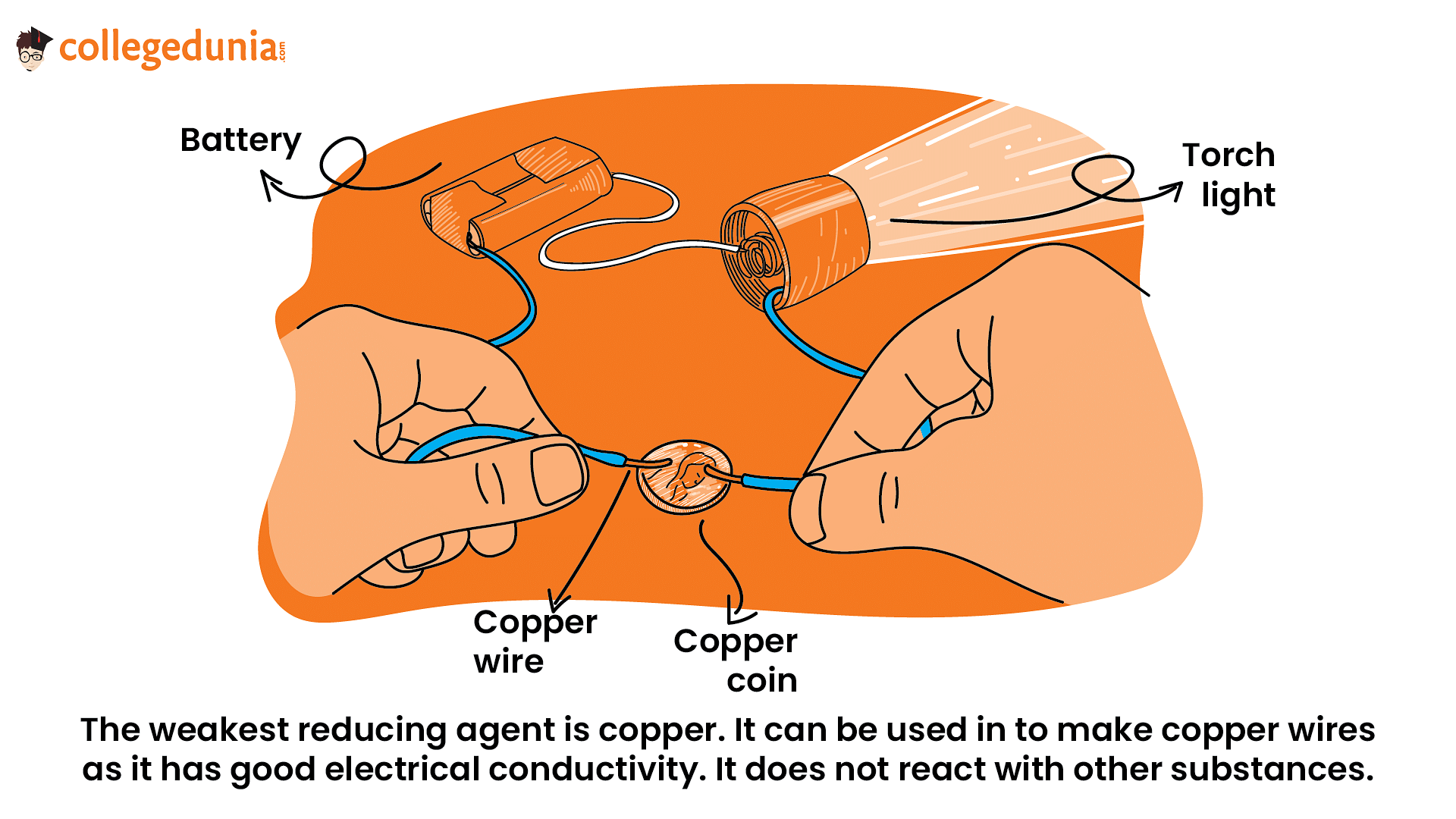
The weakest reducing agent is copper. It can be used in:
- To make copper wires as it has good electrical conductivity. It does not react with other substances.
- Copper coins are made from copper alloys that have zinc. It makes them durable.
- Copper is widely used to make marine applications as it will not react with seawater and thus is resistant to corrosion.
Question can also be asked as
- What is the weakest reducing agent among the following metals?
- How do the reducing abilities of these metals compare?
- Which metal is the most difficult to oxidize?
Approach Solution -3
The Correct Answer is (B)
Group 1 elements of the periodic table are known as Alkali metals. They are strong reducing agents. This is due to the single valence electron in the valence shell. The elements can easily lose their valence electron to acquire the noble gas configuration.
Reason for the alkali metal to act as a strong reducing agent.
Reducing agents: The elements that themselves get oxidized are called reducing agents. Due to the smaller nuclear charge and low ionization potential, they have a tendency to lose the electron easily. Thus making alkali metals strong reducing agents. As we go down from Li to Cs, the ionization potential decreases, and therefore the reducing power decreases. On the other hand, the hydration enthalpy of Sodium is the lowest thus making it the weakest reducing agent. Hydration enthalpy is the amount of energy released when one mole of ions is hydrated.
Read more
| Related Concepts | ||
|---|---|---|
| Redox reaction | Valence shell | Atom |
| Atomic mass of elements | Protons | Electrons |
Top Questions on p -Block Elements
- $\textbf{Choose the correct statements about the hydrides of group 15 elements.}$
A. The stability of the hydrides decreases in the order \(\text{NH}_3 > \text{PH}_3 > \text{AsH}_3 > \text{SbH}_3 > \text{BiH}_3\)
B. The reducing ability of the hydrides increases in the order \(\text{NH}_3 < \text{PH}_3 < \text{AsH}_3 < \text{SbH}_3 < \text{BiH}_3\)
C. Among the hydrides, \(\text{NH}_3\) is a strong reducing agent while \(\text{BiH}_3\) is a mild reducing agent.
D. The basicity of the hydrides increases in the order \(\text{NH}_3 < \text{PH}_3 < \text{AsH}_3 < \text{SbH}_3 < \text{BiH}_3\)
Choose the most appropriate from the option given below:- JEE Main - 2024
- Chemistry
- p -Block Elements
- Identify the correct statements about p-block elements and their compounds.
(A) Non metals have higher electronegativity than metals.
(B) Non metals have lower ionisation enthalpy than metals.
(C) Compounds formed between highly reactive nonmetals and highly reactive metals are generally ionic.
(D) The non-metal oxides are generally basic in nature.
(E) The metal oxides are generally acidic or neutral in nature.- JEE Main - 2024
- Chemistry
- p -Block Elements
- $\textbf{Reduction potential of ions are given below:}$
\[ \begin{array}{ccc} \text{ClO}_4^- & \text{IO}_4^- & \text{BrO}_4^- \\ E^\circ = 1.19 \, \text{V} & E^\circ = 1.65 \, \text{V} & E^\circ = 1.74 \, \text{V} \\ \end{array} \]
The correct order of their oxidising power is:- JEE Main - 2024
- Chemistry
- p -Block Elements
The number of oxygen atoms present in chemical formula of fuming sulphuric acid is _______.
- JEE Main - 2024
- Chemistry
- p -Block Elements
- Match List-I with List – II :
List-I (Oxoacids of Sulphur) List-II (Bonds) A Peroxodisulphuric acid I Two S–OH, Four S=O, One S–O–S B Sulphuric acid II Two S–OH, One S=O C Pyrosulphuric acid III Two S–OH, Four S=O, One S–O–O–S D Sulphurous acid IV Two S–OH, Two S=O Choose the correct answer from the options given below.- NEET (UG) - 2023
- Chemistry
- p -Block Elements
Questions Asked in JEE Main exam
- Let \[\vec{a} = \hat{i} + \hat{j} + \hat{k}, \quad \vec{b} = -\hat{i} - 8\hat{j} + 2\hat{k}, \quad \text{and} \quad \vec{c} = 4\hat{i} + c_2\hat{j} + c_3\hat{k} \]be three vectors such that \[\vec{b} \times \vec{a} = \vec{c} \times \vec{a}.\]If the angle between the vector $\vec{c}$ and the vector $3\hat{i} + 4\hat{j} + \hat{k}$ is $\theta$, then the greatest integer less than or equal to $\tan^2 \theta$ is:
- JEE Main - 2024
- Vector Algebra
- 10 mL of gaseous hydrocarbon on combustion gives 40 mL of CO\(_2\)(g) and 50 mL of water vapour. The total number of carbon and hydrogen atoms in the hydrocarbon is ______ .
- JEE Main - 2024
- Hydrocarbons
- If each term of a geometric progression \( a_1, a_2, a_3, \dots \) with \( a_1 = \frac{1}{8} \) and \( a_2 \neq a_1 \), is the arithmetic mean of the next two terms and \( S_n = a_1 + a_2 + \dots + a_n \), then \( S_{20} - S_{18} \) is equal to
- JEE Main - 2024
- Arithmetic Mean
A body of mass 1000 kg is moving horizontally with a velocity of 6 m/s. If 200 kg extra mass is added, the final velocity (in m/s) is:
- JEE Main - 2024
- speed and velocity
- Consider the following redox reaction:\[\text{MnO}_4^- + \text{H}^+ + \text{H}_2 \text{C}_2 \text{O}_4 \rightleftharpoons \text{Mn}^{2+} + \text{H}_2 \text{O} + \text{CO}_2\]The standard reduction potentials are given as below (E\(_\text{red}\)):\[E^\circ_{\text{MnO}_4^-/\text{Mn}^{2+}} = +1.51 \, \text{V}\]\[E^\circ_{\text{CO}_2/\text{H}_2 \text{C}_2 \text{O}_4} = -0.49 \, \text{V}\]If the equilibrium constant of the above reaction is given as \(K_\text{eq} = 10^x\), then the value of (x = ________ ) (nearest integer).
- JEE Main - 2024
- Redox reactions
Concepts Used:
P-Block Elements
- P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
- P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
P block elements consist of:
- Group 13 Elements: Boron family
- Group 14 Elements: Carbon family
- Group 15 Elements: Nitrogen family
- Group 16 Elements: Oxygen family
- Group 17 Elements: Fluorine family
- Group 18 Elements: Neon family



