Which of the following is most basic?
Tl2O3
Tl2O2
Cr2O3
B2O3
The Correct Option is B
Approach Solution - 1
Tl+ oxide is more basic than \(Tl_3\)+ \(Cr_2O_3\) is amphoteric.
Approach Solution -2
Out of Tl2O3 and Tl2O2, Tl2O2 is expected to be more basic because it has a higher oxidation state of +1 for Tl as compared to +3 in Tl2O3. Higher oxidation state of the metal in an oxide generally leads to a more basic oxide.
Therefore, Tl2O2 is the most basic among the given options.
Answer. B
Top Questions on Amines
- Replacing one hydrogen from NH3, by alkyl group will result in the formation of
- Statement-I : Aniline on reaction with concentrated \(H_2SO_4\) at 475 K gives p-amino benzene sulphonic acid. This gives blood red colour with Lassaigne’s test.
Statement-II : Aniline forms a salt with anhydrus \(AlCl_3\) in Friedel Craft’s reaction. - Pick the incorrect statement about aniline.
- Statement-1: Methyl orange is a weak acid
Statement-2: Benzenoid form of methyl orange is deeply coloured than quinonoid form
Check out which of the above statement is true? - Match the terms in Column-I with their description in Column-II and choose the correct option:
Amino Acid Letter Code (A). Alanine (P). N (B). Asparagine (Q). A (C). Aspartic acid (R). R (D). Arginine (S). D
Questions Asked in JEE Main exam
- An electron in the 5th excited state of He+ atom moves to the 1st excited state. Find the number of possible spectral lines formed.
- JEE Main - 2024
- Atomic Spectra
- In given circuit, reading of voltmeter is 1 V, then resistance of
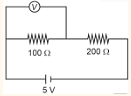
- JEE Main - 2024
- Heat Transfer
- We are given with three NaCl samples and their Van't Hoff factors. Which is correct option ?
Sample Van't Haff Factor Sample - 1 (0.1 M) \(i_1\) Sample - 2 (0.01 M) \(i_2\) Sample - 3 (0.001 M) \(i_2\) - JEE Main - 2024
- Solutions
- Complementary stand of DNA ATGCTTCA is:
- JEE Main - 2024
- Hydrogen Bonding
- Which is following compound is easily attacked by electrophile?
- JEE Main - 2024
- Organic Chemistry- Some Basic Principles and Techniques
JEE Main Notification
 JEE Main 2025 Chapter-wise Question Papers Available, Download HereSep 09, 2024
JEE Main 2025 Chapter-wise Question Papers Available, Download HereSep 09, 2024Concepts Used:
Amines - Chemical Properties
There are many chemical properties of amines.
The primary and secondary amines, including several amine derivatives, have a direct impact on their properties due to the presence of hydrogen bonding. The compounds containing phosphorus have a lower boiling point and the compounds containing amines and alcohol have a higher boiling point. The structure of alkanols is immensely similar to that of amine except the presence of the hydroxyl group. In such a case, oxygen has a higher electronegativity than that of nitrogen, so alkanol compounds are more acidic in nature in comparison to the amines.
On account of the ability to form hydrogen bonds, the amines have tendencies of high solubility in water. The amine molecules such as Ethyl, diethyl, triethyl, and Methyl are gaseous in nature. Whereas, higher weight amines have a solid structure and alkyl amines have a liquid structure. There is an ammonia smell to gaseous amines and a fishy smell to liquid amines. The solubility of amines entirely depends upon the number of carbon atoms in the molecule.



