What are the major differences between metals and non-metals?
Solution and Explanation
| Metals | Non-metals | ||
|---|---|---|---|
| 1 | Metals can lose electrons easily. | 1 | Non-metals cannot lose electrons easily. |
| 2 | Metals cannot gain electrons easily. | 2 | Non-metals cannot lose electrons easily. |
| 3 | Metals generally form ionic compounds. | 3 | Non-metals generally form covalent compounds. |
| 4 | Metals oxides are basic in nature. | 4 | Non-metals generally form covalent compounds. |
| 5 | Metals have low ionization enthalpies. | 5 | Non-metals have high ionization enthalpies |
| 6 | Metals have less negative electron gain enthalpies. | 6 | Non - metals have high negative electron gain enthalpies. |
| 7 | Metals are less electronegative. They are rather electropositive elements. | 7 | Non-metals are electronegative. |
| 8 | Metals have a high reducing power. | 8 | Non-metals have a low reducing power. |
Top Questions on Periodic Trends In Properties Of Elements
- Given below are two statements :
Statement I : Gallium is used in the manufacturing of thermometers.
Statement II : A thermometer containing gallium is useful for measuring the freezing point (256 K) of brine solution.
In the light of the above statement, choose the correct answer from the options given below :- JEE Main - 2024
- Chemistry
- Periodic Trends In Properties Of Elements
- The CORRECT order of acidity of the following compounds is
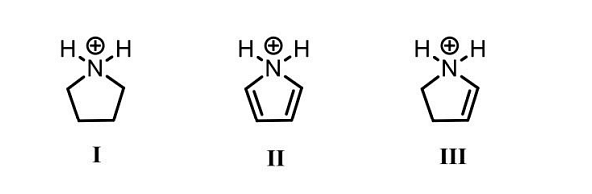
- GATE XL - 2024
- Chemistry
- Periodic Trends In Properties Of Elements
- The maximum number of electrons that can be accommodated in the shell with n = 2 is______ (in integer). (Given: n = principal quantum number)
- GATE XL - 2024
- Chemistry
- Periodic Trends In Properties Of Elements
- The CORRECT order of electronegativity is
- GATE XL - 2024
- Chemistry
- Periodic Trends In Properties Of Elements
- The CORRECT order of acidity of the following compounds is

- GATE XL - 2024
- Chemistry
- Periodic Trends In Properties Of Elements
Questions Asked in CBSE Class XI exam
- How many litres of water will have to be added to 1125 litres of the 45% solution of acid so that the resulting mixture will contain more than 25% but less than 30% acid content?
- CBSE Class XI
- inequalities
- There is mention of three communities in the story: the Marathas, the Mughals, the Anglo-Indians. Which language do you think they used within their communities and while speaking to the other groups?
- CBSE Class XI
- The adventure
- Take one flower of the family Solanaceae and write its semi-technical description. Also draw their floral diagram.
- CBSE Class XI
- The Flower
- Find the derivative of x2 – 2 at x = 10
- CBSE Class XI
- Derivatives
Figures 9.20(a) and (b) refer to the steady flow of a (non-viscous) liquid. Which of the two figures is incorrect ? Why ?

- CBSE Class XI
- Pressure
Concepts Used:
Classification of Elements & Periodicity in Properties
Since many elements were being discovered in the 19th century and the study of these elements individually was proving difficult, classification of elements was made necessary.
Classification by Johann Dobereiner - German chemist Johann Dobereiner classified certain elements on the basis of their similar properties in the groups of continuing - three elements each. These groups were called ‘triads’. In every triad, the atomic weight of the middle element was equal to the average of the atomic weights of the first and third elements.
Newlands Law of Octaves - The elements were arranged in increasing order of their atomic weights and found that every 8th element shows similarity with the 1st element.
Mendeleev’s Periodic Table - The arrangement of all 63 elements in rows or columns in order of their atomic weight was made by Mendeleev. He left some space for corresponding elements in his periodic table which were not even discovered till then. Although he predicted the properties of those elements through his periodic classification of elements.
Modern Periodic Law - The properties of the elements of the modern periodic law are periodic functions of their atomic numbers.



