Question:
The total pressure observed by mixing two liquids $A$ and $B$ is $350 \, mm$ Hg when their mole fractions are $0.7$ and $0.3$ respectively The total pressure becomes $410 \,mm \,Hg$ if the mole fractions are changed to $0.2$ and $0.8$ respectively for $A$ and $B$ The vapour pressure of pure $A$ is _________$mm \,Hg$ (Nearest integer) Consider the liquids and solutions behave ideally.
The total pressure observed by mixing two liquids $A$ and $B$ is $350 \, mm$ Hg when their mole fractions are $0.7$ and $0.3$ respectively The total pressure becomes $410 \,mm \,Hg$ if the mole fractions are changed to $0.2$ and $0.8$ respectively for $A$ and $B$ The vapour pressure of pure $A$ is _________$mm \,Hg$ (Nearest integer) Consider the liquids and solutions behave ideally.
Updated On: Sep 14, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 314
Solution and Explanation
The correct answer is 314
Let V.P. of pure be
Let V.P of pure be
When
(i)
When
(ii)
Solving (i) and (ii)
Let V.P. of pure be
Let V.P of pure be
When
(i)
When
(ii)
Solving (i) and (ii)
Was this answer helpful?
2
7
Top Questions on Solutions
- We are given with three NaCl samples and their Van't Hoff factors. Which is correct option ?
Sample Van't Haff Factor Sample - 1 (0.1 M) \(i_1\) Sample - 2 (0.01 M) \(i_2\) Sample - 3 (0.001 M) \(i_2\) - Which of the following solutions shows positive deviation from Raoult's law?
- IUPAC name of compound
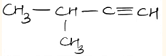
- A saturated \(CaCO_3 \)stock solution is existing at 25°C. In one experiment (i) 25 g \(Na_2CO_3 \)is added to the stock solution. In another experiment (ii) 25 g \(Na_2SO_4\) is added to the stock solution. Select the correct statement from the following.
- The ionic strength of a solution containing 0.01M of $CaCl_2$ and 0.001M of $Na_2SO_4$ is _____ M (rounded off to 3 decimal places)
View More Questions
Questions Asked in JEE Main exam
- An electron in the 5th excited state of He+ atom moves to the 1st excited state. Find the number of possible spectral lines formed.
- JEE Main - 2024
- Atomic Spectra
- In given circuit, reading of voltmeter is 1 V, then resistance of
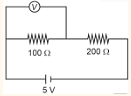
- JEE Main - 2024
- Heat Transfer
- Which is following compound is easily attacked by electrophile?
- JEE Main - 2024
- Organic Chemistry- Some Basic Principles and Techniques
- Complementary stand of DNA ATGCTTCA is:
- JEE Main - 2024
- Hydrogen Bonding
- Which of the following is correct for adiabatic free expansion against vacuum?
- JEE Main - 2024
- Thermodynamics
View More Questions
JEE Main Notification
 JEE Main 2025 Chapter-wise Question Papers Available, Download HereSep 09, 2024
JEE Main 2025 Chapter-wise Question Papers Available, Download HereSep 09, 2024JEE Main 2025 chapter-wise question papers, along with answer keys and expert solutions available here. Download the PDFs for better preparation on our website.
Concepts Used:
Solutions
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm.
For example, salt and sugar is a good illustration of a solution. A solution can be categorized into several components.
Types of Solutions:
The solutions can be classified into three types:
- Solid Solutions - In these solutions, the solvent is in a Solid-state.
- Liquid Solutions- In these solutions, the solvent is in a Liquid state.
- Gaseous Solutions - In these solutions, the solvent is in a Gaseous state.
On the basis of the amount of solute dissolved in a solvent, solutions are divided into the following types:
- Unsaturated Solution- A solution in which more solute can be dissolved without raising the temperature of the solution is known as an unsaturated solution.
- Saturated Solution- A solution in which no solute can be dissolved after reaching a certain amount of temperature is known as an unsaturated saturated solution.
- Supersaturated Solution- A solution that contains more solute than the maximum amount at a certain temperature is known as a supersaturated solution.



