Question:
The structure of a peptide is given below:

If the absolute values of the net charge of the peptide at $pH =2, pH =6$, and $pH =11$ are $\left| z _{1}\right|,\left| z _{2}\right|$, and $\left| z _{3}\right|$, respectively, then what is $\left|z_{1}\right|+\left|z_{2}\right|+\left|z_{3}\right|$ ?
The structure of a peptide is given below:

If the absolute values of the net charge of the peptide at $pH =2, pH =6$, and $pH =11$ are $\left| z _{1}\right|,\left| z _{2}\right|$, and $\left| z _{3}\right|$, respectively, then what is $\left|z_{1}\right|+\left|z_{2}\right|+\left|z_{3}\right|$ ?

If the absolute values of the net charge of the peptide at $pH =2, pH =6$, and $pH =11$ are $\left| z _{1}\right|,\left| z _{2}\right|$, and $\left| z _{3}\right|$, respectively, then what is $\left|z_{1}\right|+\left|z_{2}\right|+\left|z_{3}\right|$ ?
Updated On: May 21, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 5
Solution and Explanation
(I) At pH = 2
(highly acidic), the structure of the peptide will be as shown below.
Net charge +2
|Z1| = 2 at pH = 2
(ii) At pH = 6 (neutral solution)
In neutral medium, the given tripeptide exists as Zwitter ion.
net charge = 0
|Z2| = 0 at pH = 6
(iii)At pH = 11 (basic medium)
In basic medium the given tripeptide exists in anionic form.
Net charge = –3
So, |Z3| = |-3|
|Z1| + |Z2| + |Z3| = 2 + 0 + 3 = 5
Was this answer helpful?
0
0
Top Questions on Biomolecules
- Match List-I with List-II:

- CUET (UG) - 2024
- Chemistry
- Biomolecules
- For a double strand DNA, one strand is given below:
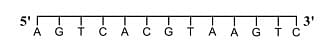
The amount of energy required to split the double strand DNA into two single strands is _____ kcal mol−1.
[Given: Average energy per H-bond for A-T base pair = 1.0 kcal mol−1, G-C base pair = 1.5 kcal mol−1, and A-U base pair = 1.25 kcal mol−1. Ignore electrostatic repulsion between the phosphate groups.]- JEE Advanced - 2024
- Chemistry
- Biomolecules
- Aspartame, an artificial sweetener, is a dipeptide aspartyl phenylalanine methyl ester. The structure of aspartame is
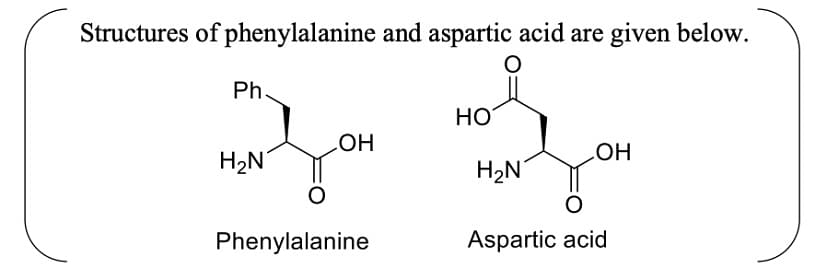
- JEE Advanced - 2024
- Chemistry
- Biomolecules
Given below are two statements:
Statement I: A unit formed by the attachment of a base to 1' position of sugar is known as nucleoside.Statement II: When nucleoside is linked to phosphorous acid at 5 -position of sugar moiety, we get neucleotide.In the light of the above statements, choose the correct answer from the options given below:- NEET (UG) - 2023
- Chemistry
- Biomolecules
- How many of the following- amino acids contain sulphur?
Lysine; Methionine; Glutamic acid; Threonine
Arginine; Cysteine; Tyrosine; Isoleucine- JEE Main - 2023
- Chemistry
- Biomolecules
View More Questions
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.

Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
View More Questions



