Question:
The standard reduction potential (E0 ) of Fe3+→Fe is____V.
[Given: Fe3+→Fe2+ E 0 = 0.77 V and
Fe2+→Fe E 0=−0.44 V]
(round off to three decimal places)
The standard reduction potential (E0 ) of Fe3+→Fe is____V.
[Given: Fe3+→Fe2+ E 0 = 0.77 V and
Fe2+→Fe E 0=−0.44 V]
(round off to three decimal places)
[Given: Fe3+→Fe2+ E 0 = 0.77 V and
Fe2+→Fe E 0=−0.44 V]
(round off to three decimal places)
Updated On: Oct 1, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: -0.036 - -0.037
Solution and Explanation
The correct answer is: ‐0.037 to -0.036 (approx)
Was this answer helpful?
0
0
Top Questions on Electrochemistry
- What pressure (bar) of \( \text{H}_2 \) would be required to make the emf of a hydrogen electrode zero in pure water at \( 25^\circ \text{C} \)?
- JEE Main - 2024
- Chemistry
- Electrochemistry

- JEE Main - 2024
- Chemistry
- Electrochemistry
- The emf of cell Tl$\vert^{Tl^+}_{_{(0.001M)}}\vert^{Cu^{2+}}_{_{(0.01M)}}$Cu is 0.83 V at 298 K. It could be increased by:
- JEE Main - 2024
- Chemistry
- Electrochemistry
- For a strong electrolyte, a plot of molar conductivity against \( (\text{concentration})^{1/2} \) is a straight line, with a negative slope, the correct unit for the slope is:
- JEE Main - 2024
- Chemistry
- Electrochemistry
- Fuel cell, using hydrogen and oxygen as fuels,
A. has been used in spaceship
B. has as efficiency of 40% to produce electricity
C. uses aluminium as catalysts
D. is eco-friendly
E. is actually a type of Galvanic cell only- JEE Main - 2024
- Chemistry
- Electrochemistry
View More Questions
Questions Asked in IIT JAM CY exam
- Exhaustive hydrogenation of the following compound
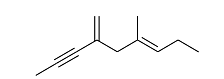
under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is _______.- IIT JAM CY - 2024
- Stereochemistry
- A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
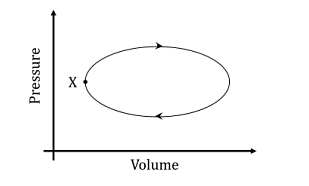
The correct statement about this process is- IIT JAM CY - 2024
- Thermodynamics
- The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:
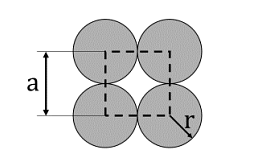
The percentage (%) area occupied by the grey circles (of radius r) inside the unit cell is _______. (rounded off to the nearest integer)- IIT JAM CY - 2024
- Solid State
- The ratio of osmotic pressures of aqueous solutions of 0.01 M BaCl2 to 0.005 M NaCl is
[Given: Both compounds dissociate completely in water]- IIT JAM CY - 2024
- Chemical equilibria
- The specific rotation of an optically pure compound is +75.3 (c 1.0 in CHCl3) at 20 °C. A synthetic sample of the same compound showed a specific rotation of +66.3 (c 1.0 in CHCl3) at 20 °C. The enantiomeric excess (ee) of the synthetic sample is _______ %.
(rounded off to the nearest integer)- IIT JAM CY - 2024
- Stereochemistry
View More Questions



