Question:
The Lewis acid character of boron tri halides follows the order :
The Lewis acid character of boron tri halides follows the order :
Updated On: Sep 09, 2024
- $BCl _3> BF _3> BBr _3> BI _3$
- $BI _3> BBr _3> BCl _3> BF _3$
- $BBr _3> BI _3> BCl _3> BF _3$
- $BF _3> BCl _3> BBr _3> BI _3$
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
Extent of back bonding, reduces down the group leading to more Lewis acidic strength
So, the correct option is (B).
So, the correct option is (B).
Was this answer helpful?
0
0
Top Questions on p -Block Elements
- Arrange the following according to their decreasing oxidizing power : BrO4-, IO4-, CIO4-
- JEE Main - 2024
- Chemistry
- p -Block Elements
- Which of the following represents the correct order of metallic character of the given elements?
- JEE Main - 2023
- Chemistry
- p -Block Elements
- Match List I with List II Column I(Molecule/ions)Column III (No of lone pairs of $e^-$ on central metal atom)A$ IF_7$iThreeB$ICl_4^-$iiOneC$XeF_6$iiiTwoD$ XeF_2 $ivZero Choose the correct answer from the options given below:
- JEE Main - 2023
- Chemistry
- p -Block Elements
- Match List I with List II
LIST I LIST II A Chlorophyll 1 \(Na _2 CO _3\) B Soda ash 2 \(CaSO _4\) C Dentistry, Ornamental work 3 \(Mg ^{2+}\) D Used in white washing 4 \(Ca ( OH )_2\) - JEE Main - 2023
- Chemistry
- p -Block Elements
- Sum of $\pi$-bonds present in peroxodisulphuric acid and pyrosulphuric acid is___________
- JEE Main - 2023
- Chemistry
- p -Block Elements
View More Questions
Questions Asked in JEE Main exam
- An electron in the 5th excited state of He+ atom moves to the 1st excited state. Find the number of possible spectral lines formed.
- JEE Main - 2024
- Atomic Spectra
- In given circuit, reading of voltmeter is 1 V, then resistance of
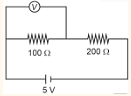
- JEE Main - 2024
- Heat Transfer
- We are given with three NaCl samples and their Van't Hoff factors. Which is correct option ?
Sample Van't Haff Factor Sample - 1 (0.1 M) \(i_1\) Sample - 2 (0.01 M) \(i_2\) Sample - 3 (0.001 M) \(i_2\) - JEE Main - 2024
- Solutions
- Complementary stand of DNA ATGCTTCA is:
- JEE Main - 2024
- Hydrogen Bonding
- Which is following compound is easily attacked by electrophile?
- JEE Main - 2024
- Organic Chemistry- Some Basic Principles and Techniques
View More Questions
JEE Main Notification
 JEE Main 2025 Chapter-wise Question Papers Available, Download HereSep 09, 2024
JEE Main 2025 Chapter-wise Question Papers Available, Download HereSep 09, 2024JEE Main 2025 chapter-wise question papers, along with answer keys and expert solutions available here. Download the PDFs for better preparation on our website.
Concepts Used:
P-Block Elements
- P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
- P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
P block elements consist of:
- Group 13 Elements: Boron family
- Group 14 Elements: Carbon family
- Group 15 Elements: Nitrogen family
- Group 16 Elements: Oxygen family
- Group 17 Elements: Fluorine family
- Group 18 Elements: Neon family



