Comprehension
The entropy versus temperature plot for phases \(\alpha\) and \(\beta\) at 1 bar pressure is given. \(S_T\) and \(S_0\) are entropies of the phases at temperatures \(T\) and \(0K\), respectively.

The transition temperature for \(\alpha\) to \(\beta\) phase change is \(600 K\) and \(C_P\), \(β − C_P\), \(\alpha = 1 \text{ J }mol^{−1} K^{-1}\). Assume \((C_P,\beta − C_P,\alpha)\) is independent of temperature in the range of \(200\) to \(700 K\). \(C_P,\alpha \text{ and }C_P,\beta\) are heat capacities of \(\alpha\) and \(\beta\) phases, respectively

The transition temperature for \(\alpha\) to \(\beta\) phase change is \(600 K\) and \(C_P\), \(β − C_P\), \(\alpha = 1 \text{ J }mol^{−1} K^{-1}\). Assume \((C_P,\beta − C_P,\alpha)\) is independent of temperature in the range of \(200\) to \(700 K\). \(C_P,\alpha \text{ and }C_P,\beta\) are heat capacities of \(\alpha\) and \(\beta\) phases, respectively
Question: 1
The value of enthalpy change, Hβ − Hα (in J mol−1), at 300 K is ___.
The value of enthalpy change, Hβ − Hα (in J mol−1), at 300 K is ___.
Updated On: May 24, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 300
Solution and Explanation
The enthalpy change (Hβ−Hα) at 300 K is __ J mol−1−1.
Was this answer helpful?
0
0
Question: 2
The value of entropy change, Sβ − Sα (in J mol−1 K−1), at 300 K is ___.
[Use: ln 2 = 0.69 ,Given: Sβ − Sα = 0 at 0 K]
The value of entropy change, Sβ − Sα (in J mol−1 K−1), at 300 K is ___.
[Use: ln 2 = 0.69 ,Given: Sβ − Sα = 0 at 0 K]
[Use: ln 2 = 0.69 ,Given: Sβ − Sα = 0 at 0 K]
Updated On: May 24, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 0.31
Solution and Explanation
The correct answer is 0.31
Was this answer helpful?
0
0
Top Questions on Thermodynamics
- Which of the following is correct for adiabatic free expansion against vacuum?
- JEE Main - 2024
- Chemistry
- Thermodynamics
- In an air-standard Otto cycle, the pressure and temperature of air just before the compression stroke are 200 kPa and 26.85 °C, respectively. The combustion process is assumed to be a constant volume process, where 1.02 MJ/kg heat is added. The cycle efficiency is 50%. The adiabatic index \(γ\) and specific heat at constant volume \(C_v\) can be considered to be constant during the process (corresponding values taken at the mean cycle temperature).
Assuming that the ideal gas law is applicable, \(γ = \frac 43\) and \(c_v= 0.85\ kJ/kg-K\), the maximum pressure (in MPa) reached during the cycle is _____ . (Rounded off to 1 decimal place)- GATE PI - 2024
- General Engineering
- Thermodynamics
- Match List I with List II
List-I
(Process)List-II
(Conditions)(A) Isothermal process (I) No heat exchange (B) Isochoric process (II) Carried out at constant temperature (C) Isobaric process (III) Carried out at constant volume (D) Adiabatic process (IV) Carried out at constant pressure
Choose the correct answer from the options given below:- NEET (UG) - 2024
- Chemistry
- Thermodynamics
- Consider the two statements (Assume density of water to be constant):
Statement 1: A capillary tube is first dipped in hot water and then dipped in cold water. The rise is higher in hot water.
Statement 2: Capillary tube is first dipped in cold water and then in hot water. The rise is higher in cold water.- JEE Main - 2024
- Physics
- Thermodynamics
- The Pressure (P) versus volume (V) of thermodynamic process shown in figure. The select the correct options (Take γ= 1.1)
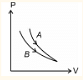
- JEE Main - 2024
- Physics
- Thermodynamics
View More Questions
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
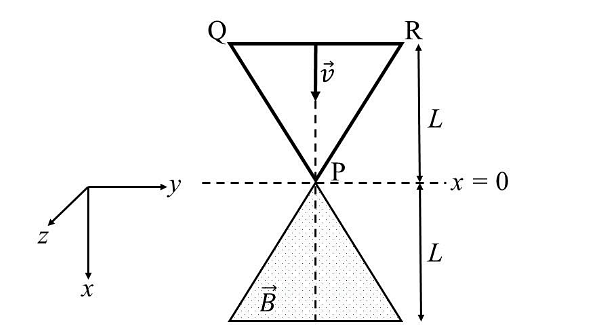
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
View More Questions



