Question:
The Cu metal crystallises into fcc lattice with a unit cell edge length of 361 pm. The radius of Cu atom is:
The Cu metal crystallises into fcc lattice with a unit cell edge length of 361 pm. The radius of Cu atom is:
Updated On: Oct 29, 2024
- 127 pm
- 181 pm
- 157 pm
- 108 pm
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
\(\text{In a face-centered cubic (fcc) lattice, the relation between the unit cell edge length } a \text{ and the atomic radius } r \text{ is:} \\ r = \frac{a}{2\sqrt{2}} \\ \text{Substituting } a = 361 \, \text{pm:} \\ r = \frac{361}{2\sqrt{2}} \approx 127 \, \text{pm}\)
Was this answer helpful?
0
0
Top Questions on Solid State
- Which among the following compounds show metal excess defect due to anionic vacancy?
- CUET (UG) - 2024
- Chemistry
- Solid State
- Dry ice is:
- CUET (UG) - 2024
- Chemistry
- Solid State
- Which among the following is a supercooled liquid?
- CUET (UG) - 2024
- Chemistry
- Solid State
- The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:
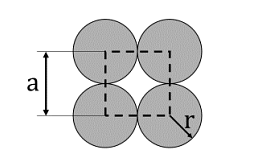
The percentage (%) area occupied by the grey circles (of radius r) inside the unit cell is _______. (rounded off to the nearest integer) - Wavelength of X-rays used in a diffraction experiment is 1.54 Å. X-rays are diffracted from a set of planes with an interplanar spacing of 1.54 Å. Then the angle θ (in degrees) corresponding to the first-order Bragg diffraction is
View More Questions
Questions Asked in CUET exam
- If the energy of incident radiation is increased by $25\%$, the kinetic energy of the photoelectrons emitted increases from 0.6 eV to 0.9 eV. The work function of the metal is:
- CUET (UG) - 2024
- Dual nature of matter
- Read the following passage and answer the next five questions based on it.
Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons. Sterically, the presence of two large groups in ketones hinders the attack of nucleophile to carbonyl carbon than in aldehydes. Electronically, aldehydes are more reactive than ketones because two alkyl groups reduce the electrophilicity of the carbonyl carbon more effectively than in the former- CUET (UG) - 2024
- Aldehydes, Ketones and Carboxylic Acids
- Match List-I with List-II:
List-I List-II (A) Mn2+ (I) Pyrolusite ore (B) Spin only Magnetic Moment (II) An alloy of 4f metal, iron and traces of S, C, Al and Ca (C) MnO2 (III) μs = √n(n + 2) BM (D) Misch metal (IV) Highest oxidation states
Choose the correct answer from the options given below:- CUET (UG) - 2024
- Magnetic moment
- Read the following passage and answer the next five questions based on it.
The transition metals are very hard and have low volatility. Their melting and boiling points are high. In any row, the melting points of these metals rise to a maximum at d5 and fall regularly as atomic number increases. The high melting points of these metals are attributed to the involvement of greater number of electrons from (n– 1)d in addition to ns electrons in the interatomic metallic bonding.- CUET (UG) - 2024
- General Properties of the Transition Elements (d-Block)
- Match List-I with List-II:

Choose the correct answer from the options given below:
View More Questions



