The compound which contains both ionic and covalent
bonds is
- $CH_4$
- $H_2$
- KCN
- KCl
The Correct Option is C
Solution and Explanation
is ionic while carbon and nitrogen are covalently bonded in
cyanide ion as:
Top Questions on Polarity of bonds
- Consider the following sequence of reactions:
 .Select A and B respectively.
.Select A and B respectively.- JEE Main - 2024
- Chemistry
- Polarity of bonds
- Which of the following is a Polar molecule?
- JEE Main - 2024
- Chemistry
- Polarity of bonds
- Which one of the following pairs is an example of polar molecular solids ?
- JEE Main - 2023
- Chemistry
- Polarity of bonds
- Polar stratospheric clouds facilitate the formation of:
- JEE Main - 2022
- Chemistry
- Polarity of bonds
- The most polar compound among the following is :
- JEE Main - 2018
- Chemistry
- Polarity of bonds
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
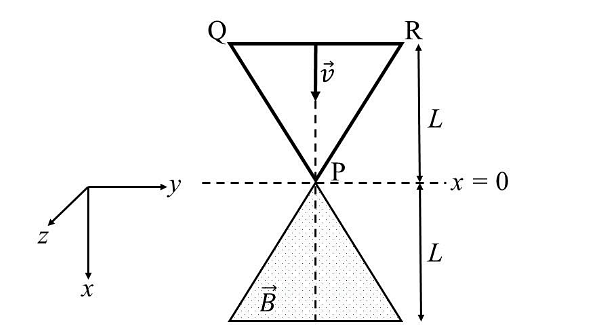
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
Concepts Used:
Polarity of bonds
Polarity, in chemical bonding, distribution of electrical charge over the atoms joined by the bond. Specifically, while bonds between identical atoms, as in H2, are electrically uniform in the sense that both hydrogen atoms are electrically neutral, bonds between atoms of different elements are electrically inequivalent.
Non-polar Covalent Bond:
A non-polar covalent bond is a type of chemical bond that is formed when electrons are shared equally between two atoms. Thus, in an atom, the number of electrons shared by the adjacent atoms will be the same.
The covalent bond is also termed as nonpolar because the difference in electronegativity is mostly negligible.
Polar Covalent Bond:
A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. Consider the hydrogen chloride (HCl) molecule. Each atom in HCl requires one more electron to form an inert gas electron configuration.
Dipole Moment
Dipole moment is defined as the product of the magnitude of charge and the distance of separation between the centres of positive and negative charge.
Dipole moment is a vector quantity and it is denoted by µ.
µ = charge (Q) * Distance of separation(r)
The dipole moment is expressed in Debye units (D).



