The Cannizzaro's reaction is not given by
- trimethyl acetaldehyde
- acetaldehyde
- benzaldehyde
- fonnaldehyde
The Correct Option is B
Solution and Explanation
Top Questions on Chemical Reactions
- The rate of a reaction quadruples when temperature changes from 27°c to 57°c. Calculate the energy of activation
Given R=8.314 J K-1 mol-1, log 4=0.6021- NEET (UG) - 2024
- Chemistry
- Chemical Reactions
- For given reaction:
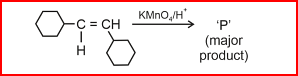
‘P’ is- NEET (UG) - 2024
- Chemistry
- Chemical Reactions
- Identify the correct reagents that would bring about the following transformation.
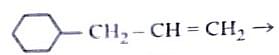
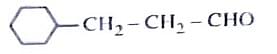
- NEET (UG) - 2024
- Chemistry
- Chemical Reactions
- Identify the major product C formed in the following reaction sequence:
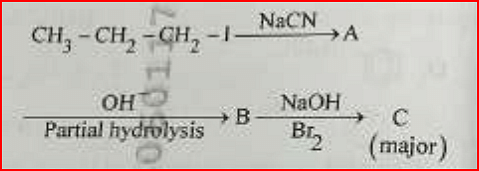
- NEET (UG) - 2024
- Chemistry
- Chemical Reactions
- The products A and B obtained int he following reactions, respectively, are
3ROH+PCI3→3RCI+A
ROH+PCI5→RCI+HCI+B- NEET (UG) - 2024
- Chemistry
- Chemical Reactions
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.

Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
Concepts Used:
Haloalkanes and Haloarenes - Chemical Reactions
Chemical Reactions go with the breaking and bonding of covalent bonds which involve of exchange of electrons. The functional groups of Organic compounds play a consequential role in the process. Based on the above theory, reactions can be classified into five main groups:
Rearrangement Reactions are the type of reactions in which products get formed simply by the rearrangement of atoms and electrons in the reactant molecules.
O
||
NH4CNO → NH2 –C – NH2
Substitution Reactions are the reactions in which an atom or group of atoms is replaced by some other atom or group of atoms without any change in the structure of the remaining part of the molecule.
CH3Br + KOH (aqueous) → CH3OH + KBr
Addition Reactions are the reactions in which products get formed by the addition of some reagent to an unsaturated compound.
CH2 = CH2 + HCl → CH5Cl
- Electrophilic Addition Reactions
- Nucleophilic Addition Reactions
- Free Radical Addition Reactions
Elimination Reactions are the reactions in which the products get formed by the loss of simple molecules like HX from the reactant molecules.
C2H5OH → C2H4
- EN1 (Nucleophilic Elimination Unimolecular)
- EN2 (Nucleophilic Elimination Bimolecular)
A polymerization Reaction is the union of two or more molecules of a substance that form a single molecule with higher molecular weight.
n (CH = CH2) → (-CH2 – CH2 -) n



