Question:
Passing $H_2 S$ gas into a mixture of $Mn^{2+}, Ni^{2+}, Cu^{2+}$ and $Hg^{2+}$ ions in an acidified aqueous solution precipitates
Passing $H_2 S$ gas into a mixture of $Mn^{2+}, Ni^{2+}, Cu^{2+}$ and $Hg^{2+}$ ions in an acidified aqueous solution precipitates
Updated On: Jun 14, 2022
- CuS and HgS
- MnS and CuS
- MnS and NiS
- NiS and HgS
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
In acidic medium, $H_2 S$ is very feebly ionised giving very small concentration of sulphide ion for precipitation. Therefore, the most insoluble salts CuS and HgS are precipitated only.
Was this answer helpful?
0
0
Top Questions on Organic Chemistry- Some Basic Principles and Techniques
- The set of meta directing functional groups from the following sets is:
- JEE Main - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- As per Bronsted-Lowry concept, acid is defined as:
- GPAT - 2024
- Organic Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- The correct statements among the following, for a "chromatography" purification method is:
- JEE Main - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- Which among the following is incorrect statement?
- JEE Main - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- Molar mass of the salt from NaBr, \(NaNO_3\), KI and \(CaF_2\) which does not evolve coloured vapours on heating with concentrated \(H_2SO_4\) is _____ g \(mol^{−1}\). (Molar mass in g mol−1: Na : 23, N : 14, K : 39, O : 16, Br : 80, I : 127, F : 19, Ca : 40)
- JEE Main - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
View More Questions
Questions Asked in JEE Advanced exam
- A closed vessel contains 10 g of an ideal gas X at 300 K, which exerts 2 atm pressure. At the same temperature, 80 g of another ideal gas Y is added to it and the pressure becomes 6 atm. The ratio of root mean square velocities of X and Y at 300 K is
- JEE Advanced - 2024
- States of matter
- Let the function \(f:[1,\infin)→\R\) be defined by
\(f(t) = \begin{cases} (-1)^{n+1}2, & \text{if } t=2n-1,n\in\N, \\ \frac{(2n+1-t)}{2}f(2n-1)+\frac{(t-(2n-1))}{2}f(2n+1) & \text{if } 2n-1<t<2n+1,n\in\N. \end{cases}\)
Define \(g(x)=\int\limits_{1}^{x}f(t)dt,x\in(1,\infin).\) Let α denote the number of solutions of the equation g(x) = 0 in the interval (1, 8] and \(β=\lim\limits_{x→1+}\frac{g(x)}{x-1}\). Then the value of α + β is equal to _____.- JEE Advanced - 2024
- Integral Calculus
- A dimensionless quantity is constructed in terms of electronic charge \(e\), permittivity of free space \(\epsilon_0\) , Planck’s constant ℎ, and speed of light c. If the dimensionless quantity is written as \(e^\alpha\epsilon_0^\beta h^\gamma c^\delta\)and n is a non-zero integer, then\((\alpha, \beta,\gamma,\delta)\) is given by
- JEE Advanced - 2024
- Semiconductor electronics: materials, devices and simple circuits
- A block of mass \(5 kg\) moves along the \(x-\)direction subject to the force \(F = (−20x + 10) N,\) with the value of \(x \) in metre. At time \(t = 0 s,\) it is at rest at position \(x = 1 m\). The position and momentum of the block at \(t = (\pi/4)\) s are
- JEE Advanced - 2024
- Work-energy theorem
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
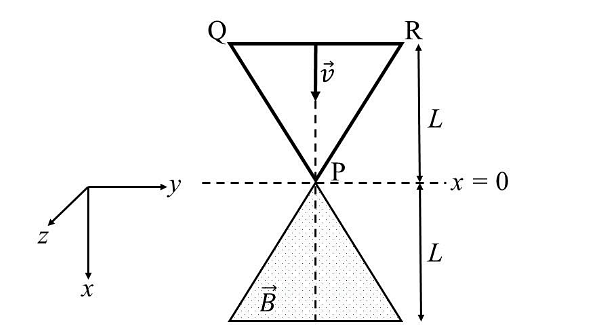
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0?
View More Questions



