Osmotic pressure can be increased by
Show Hint
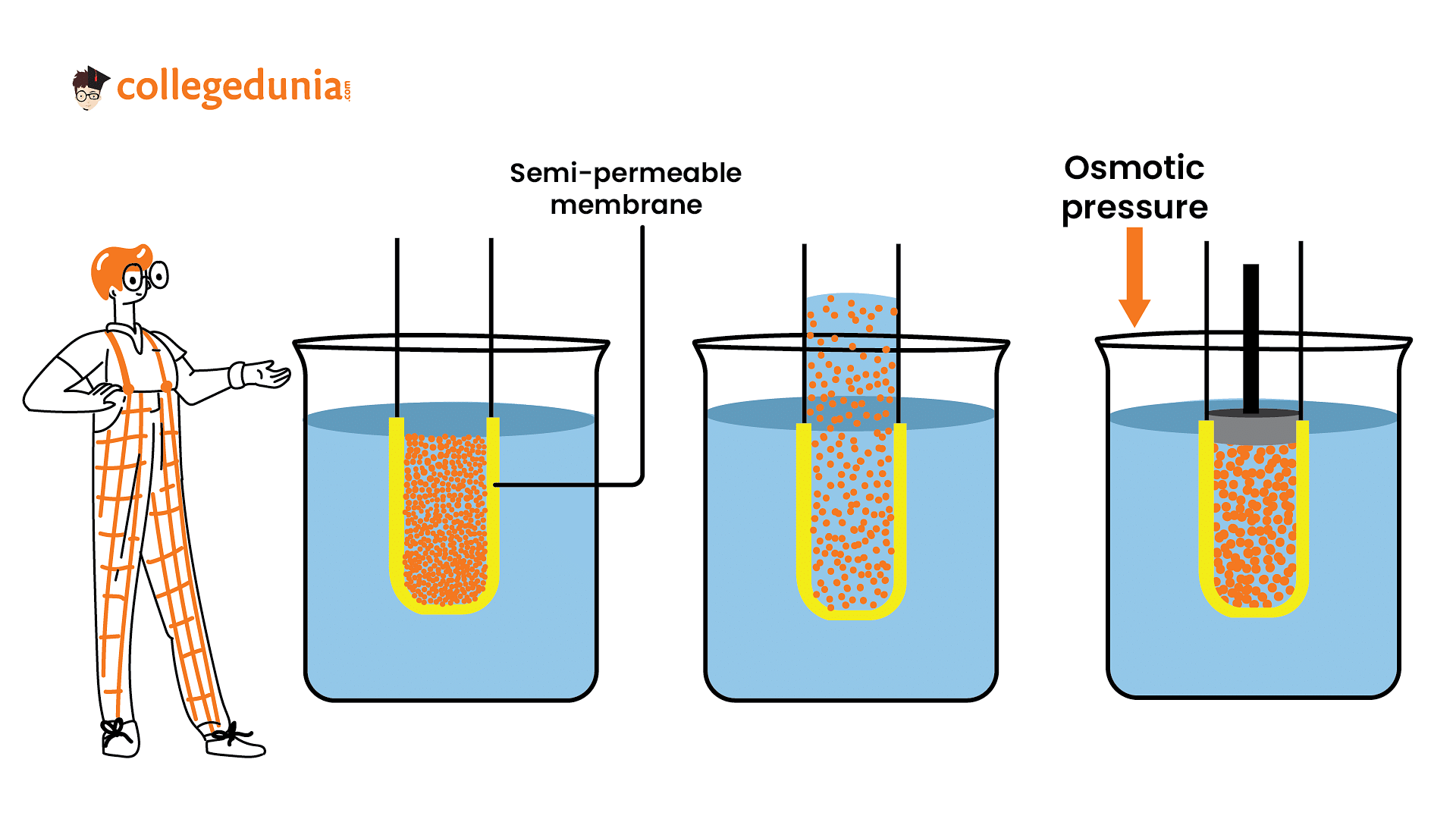
Osmotic pressure describes the pressure exerted by a solvent to prevent the influx of solvent molecules through a semipermeable membrane
- increasing the temperature of the solution.
- decreasing the temperature of the solution.
- increasing the volume of the vessel.
- diluting the solution.
The Correct Option is A
Approach Solution - 1
Osmotic pressure is directly proportional to the temperature $\because \pi=CRT$ where,
- C= concentration
- R= gas constant
- T= temperature
i.e. $\pi \propto T$, thus, on increasing the temperature, osmotic pressure also increases.
Discover More From Chapter: Solutions
Approach Solution -2
Osmotic pressure describes the pressure exerted by a solvent to prevent the influx of solvent molecules through a semipermeable membrane. The temperature of a solution has a significant influence on its osmotic pressure.
Temperature and Molecular Kinetics
- Temperature is a measure of the average kinetic energy of molecules in a solution.
- An increase in temperature corresponds to an increase in molecular kinetic energy.
Effect of Temperature on Solvent-Solute Interactions
- When the temperature of a solution increases, the kinetic energy of solvent and solute molecules increases as well.
- This increased kinetic energy leads to more frequent and energetic collisions between solvent and solute particles.
Read More:
| Related Concepts | ||
|---|---|---|
| Spore Formation | Vegetative Bud | Electroosmosis |
| Amoeba | Binomial Nomenclature | Cell Wall and Cell Membrane |
Enhanced Solvent-Solute Interaction
- As the temperature rises, solvent molecules gain energy to overcome intermolecular forces.
- This enhanced interaction increases the rate of solvent molecules crossing the semipermeable membrane.
Increased Movement of Solvent
- With higher temperatures, solvent molecules move more rapidly, resulting in a greater tendency to move across the membrane.
- Consequently, the osmotic pressure of the solution increases due to the greater flow of solvent molecules.
Relationship Between Temperature and Osmotic Pressure
- Increasing the temperature of a solution increases the kinetic energy and movement of solvent molecules.
- This increased movement elevates the osmotic pressure, as more solvent molecules attempt to equalize the concentration on both sides of the semipermeable membrane.
The temperature of a solution plays a crucial role in its osmotic pressure. Increasing the temperature leads to enhanced molecular movement and more vigorous solvent-solute interactions, resulting in an increase in osmotic pressure.
Top Questions on Solutions
- We are given with three NaCl samples and their Van't Hoff factors. Which is correct option ?
Sample Van't Haff Factor Sample - 1 (0.1 M) \(i_1\) Sample - 2 (0.01 M) \(i_2\) Sample - 3 (0.001 M) \(i_2\) - Which of the following solutions shows positive deviation from Raoult's law?
- IUPAC name of compound
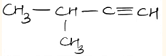
- A saturated \(CaCO_3 \)stock solution is existing at 25°C. In one experiment (i) 25 g \(Na_2CO_3 \)is added to the stock solution. In another experiment (ii) 25 g \(Na_2SO_4\) is added to the stock solution. Select the correct statement from the following.
- The ionic strength of a solution containing 0.01M of $CaCl_2$ and 0.001M of $Na_2SO_4$ is _____ M (rounded off to 3 decimal places)
Questions Asked in KCET exam
- The current in a coil changes from 2A to 5A in 0.3s. The magnitude of emf induced in the coil is 1.0V. The value of self-inductance of the coil is
- KCET - 2023
- Electromagnetic induction
- A stretched wire of a material whose Young's modulus Y = 2 × 1011 Nm-2 has Poisson's ratio of 0.25. Its lateral strain εl = 10-3. The elastic energy density of the wire is
- KCET - 2023
- mechanical properties of solids
- A particle moves along the curve \(\frac{x^2}{16}+\frac{y^2}{4}=1\). When the rate of change of abscissa is 4 times that of its ordinate, then the quadrant in which the particle lies is
- KCET - 2023
- Conic sections
- A point object is moving at a constant speed of 1 ms-1 along the principal axis of a convex lens of focal length 10cm. The speed of the image is also 1 ms-1 , when the object is at _______ cm from the optic centre of the lens.
- KCET - 2023
- spherical lenses
- A cubical Gaussian surface has side of length a = 10 cm. Electric field lines are parallel to x-axis as shown. The magnitudes of electric fields through surfaces ABCD and EFGH are 6kNC-1 and 9kNC-1 respectively. Then the total charge enclosed by the cube is
[Take ε0 = 9 × 10-12 Fm-1]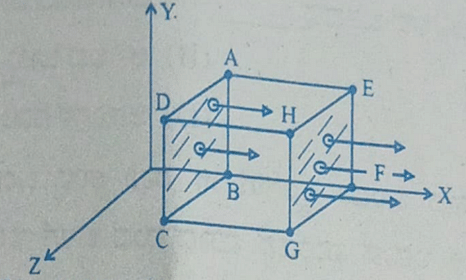
- KCET - 2023
- Gauss Law
Concepts Used:
Solutions
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm.
For example, salt and sugar is a good illustration of a solution. A solution can be categorized into several components.
Types of Solutions:
The solutions can be classified into three types:
- Solid Solutions - In these solutions, the solvent is in a Solid-state.
- Liquid Solutions- In these solutions, the solvent is in a Liquid state.
- Gaseous Solutions - In these solutions, the solvent is in a Gaseous state.
On the basis of the amount of solute dissolved in a solvent, solutions are divided into the following types:
- Unsaturated Solution- A solution in which more solute can be dissolved without raising the temperature of the solution is known as an unsaturated solution.
- Saturated Solution- A solution in which no solute can be dissolved after reaching a certain amount of temperature is known as an unsaturated saturated solution.
- Supersaturated Solution- A solution that contains more solute than the maximum amount at a certain temperature is known as a supersaturated solution.



