Match the reactions in List-I with the features of their products in List-II and choose the correct option
List-I List-II (P) (-)-1-Bromo-2-ethylpentane 
(single enantiomer) (1) Inversion of configuration (Q) (-)-2-Bromopentane 
(single enantiomer) (2) Retention of configuration (R) (-)-3- Bromo-3-methylhexane 
(single enantiometer) (3) Mixture of enantiomers (S) 

(single enantiometer)(4) Mixture of structural isomers (5) Mixture of diastereomers
| List-I | List-II | ||
| (P) | (-)-1-Bromo-2-ethylpentane  (single enantiomer) | (1) | Inversion of configuration |
| (Q) | (-)-2-Bromopentane  (single enantiomer) | (2) | Retention of configuration |
| (R) | (-)-3- Bromo-3-methylhexane  (single enantiometer) | (3) | Mixture of enantiomers |
| (S) |   (single enantiometer) | (4) | Mixture of structural isomers |
| (5) | Mixture of diastereomers |
Show Hint
(P) Configuration at chiral carbon is same.
P → 2 [reaction does not occur at chiral carbon]
(Q) Configuration at chiral carbon changes.
Q → 1
(R) SN1 → Mixture of enantiomers formed.
R → 3
(S):Refer the Image below

∴ So mixture of diastereomers are formed.
S → 5
The correct Answer is option is (B): P →2; Q→1 ; R →3; S →5
P →1; Q→2 ; R →5; S →3
P →2; Q→1 ; R →3; S →5
P →1; Q→2; R →5; S →4
P →2; Q→4; R →3; S →5
The Correct Option is B
Solution and Explanation
The correct option is (B): P →2; Q→1 ; R →3; S →5
Top Questions on Stoichiometry and Stoichiometric Calculations
- 9.3 g of pure aniline is treated with bromine water at room temperature to give a white precipitate of the product 'P'. The mass of product 'P' obtained is 26.4 g. The percentage yield is ......... %.
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- \( X \, \text{g} \) of ethylamine is subjected to reaction with \( \text{NaNO}_2/\text{HCl} \) followed by water; evolved dinitrogen gas which occupied \( 2.24 \, \text{L} \) volume at STP. \( X \) is ______ \( \times 10^{-1} \, \text{g} \).
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- If 50 mL of 0.5 M oxalic acid is required to neutralise 25 mL of NaOH solution, the amount of NaOH in 50 mL of given NaOH solution is _____g.
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- Consider the following reaction:\[3\text{PbCl}_2 + 2(\text{NH}_4)_3\text{PO}_4 \rightarrow \text{Pb}_3(\text{PO}_4)_2 + 6\text{NH}_4\text{Cl}\]If 72 mmol of PbCl\(_2\) is mixed with 50 mmol of (\(\text{NH}_4\))\(_3\)PO\(_4\), then the amount of \(\text{Pb}_3(\text{PO}_4)_2\) formed is ______ mmol (nearest integer).
- JEE Main - 2024
- Chemistry
- Stoichiometry and Stoichiometric Calculations
- The strength of $50$ volume solution of hydrogen peroxide is _____$g / L$ (Nearest integer)
Given: Molar mass of $H _2 O _2$ is $34\, g \, mol ^{-1}$ Molar volume of gas at $STP =22.7\, L$- JEE Main - 2023
- Chemistry
- Stoichiometry and Stoichiometric Calculations
Questions Asked in JEE Advanced exam
- Let the function \(f:[1,\infin)→\R\) be defined by
\(f(t) = \begin{cases} (-1)^{n+1}2, & \text{if } t=2n-1,n\in\N, \\ \frac{(2n+1-t)}{2}f(2n-1)+\frac{(t-(2n-1))}{2}f(2n+1) & \text{if } 2n-1<t<2n+1,n\in\N. \end{cases}\)
Define \(g(x)=\int\limits_{1}^{x}f(t)dt,x\in(1,\infin).\) Let α denote the number of solutions of the equation g(x) = 0 in the interval (1, 8] and \(β=\lim\limits_{x→1+}\frac{g(x)}{x-1}\). Then the value of α + β is equal to _____.- JEE Advanced - 2024
- Integral Calculus
- A dimensionless quantity is constructed in terms of electronic charge \(e\), permittivity of free space \(\epsilon_0\) , Planck’s constant ℎ, and speed of light c. If the dimensionless quantity is written as \(e^\alpha\epsilon_0^\beta h^\gamma c^\delta\)and n is a non-zero integer, then\((\alpha, \beta,\gamma,\delta)\) is given by
- JEE Advanced - 2024
- Semiconductor electronics: materials, devices and simple circuits
- A block of mass \(5 kg\) moves along the \(x-\)direction subject to the force \(F = (−20x + 10) N,\) with the value of \(x \) in metre. At time \(t = 0 s,\) it is at rest at position \(x = 1 m\). The position and momentum of the block at \(t = (\pi/4)\) s are
- JEE Advanced - 2024
- Work-energy theorem
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
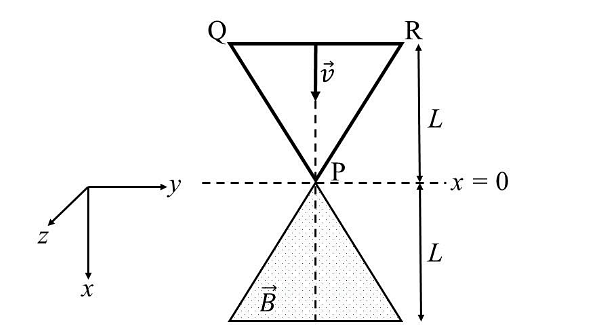
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
Concepts Used:
Stoichiometry
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated.
Stoichiometry helps us determine how much substance is needed or is present. Things that can be measured are;
- Reactants and Products mass
- Molecular weight
- Chemical equations
- Formulas
Stoichiometric Coefficient
The Stoichiometric coefficient of any given component is the number of molecules and/or formula units that participate in the reaction as written.
Mole Ratios
The mass of one mole of a substance in grams is called molar mass. The molar mass of one mole of a substance is numerically equal to the atomic/molecular formula mass.



