In the above reaction, 5 g of toluene is converted into benzaldehyde with 92% yield. The amount of benzaldehyde produced is______ × 10–2 g. (Nearest integer).
Correct Answer: 530
Solution and Explanation
Moles = \(\frac{5}{92}\)
Moles of benzaldehyde produced = \(\frac{5}{92}\)×0.92
= 0.05
= 0.05×106
= 5.3g
= 530×10-2

Top Questions on Biomolecules
- Match List-I with List-II:

- CUET (UG) - 2024
- Chemistry
- Biomolecules
- For a double strand DNA, one strand is given below:
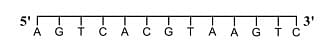
The amount of energy required to split the double strand DNA into two single strands is _____ kcal mol−1.
[Given: Average energy per H-bond for A-T base pair = 1.0 kcal mol−1, G-C base pair = 1.5 kcal mol−1, and A-U base pair = 1.25 kcal mol−1. Ignore electrostatic repulsion between the phosphate groups.]- JEE Advanced - 2024
- Chemistry
- Biomolecules
- Aspartame, an artificial sweetener, is a dipeptide aspartyl phenylalanine methyl ester. The structure of aspartame is
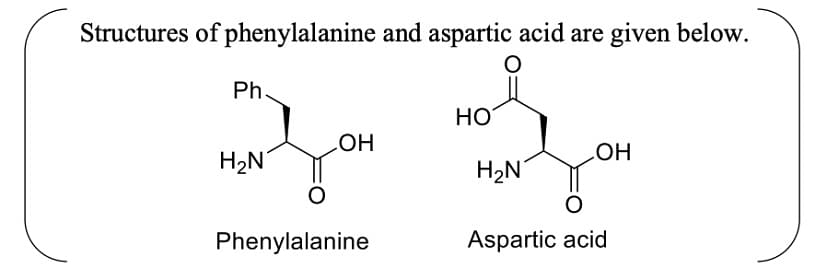
- JEE Advanced - 2024
- Chemistry
- Biomolecules
Given below are two statements:
Statement I: A unit formed by the attachment of a base to 1' position of sugar is known as nucleoside.Statement II: When nucleoside is linked to phosphorous acid at 5 -position of sugar moiety, we get neucleotide.In the light of the above statements, choose the correct answer from the options given below:- NEET (UG) - 2023
- Chemistry
- Biomolecules
- How many of the following- amino acids contain sulphur?
Lysine; Methionine; Glutamic acid; Threonine
Arginine; Cysteine; Tyrosine; Isoleucine- JEE Main - 2023
- Chemistry
- Biomolecules
Questions Asked in JEE Main exam
- Find the acceleration of \(2\) \(kg\) block shown in the diagram (neglect friction)

- JEE Main - 2024
- Acceleration
- If \(|2A|^3 =21\) and \(\begin{bmatrix} 1 & 0 & 0 \\[0.3em] 0 & α & β \\[0.3em] 0 & β & α \end{bmatrix}\), then a is (if \(α,β∈I\))
- JEE Main - 2024
- Matrices
- Let \(α\) and \(β\) the roots of equation \(px^2 + qx - r = 0\), where \(P≠ 0\). If \(p,q,r\) be the consecutive term of non constant G.P and \(\frac{1}{α} + \frac{1}{β} = \frac{3}{4}\) then the value of \((α - β)^2\) is:
- JEE Main - 2024
- Geometric Progression
- Two lines \(L_1 \;\& \;L_2\) passing through origin trisecting the line segment intercepted by the line \(4x + 5y = 20\) between the coordinate axes. Then the tangent of angle between the lines \(L_1\) and \(L_2\) is
- JEE Main - 2024
- Tangents and Normals
- Rank of the word 'GTWENTY' in dictionary is _____ .
- JEE Main - 2024
- permutations and combinations
Concepts Used:
Intermolecular Forces
The attractive and repulsive forces that arise between the molecules of a substance are termed as the intermolecular forces. These forces are responsible for the physical and chemical properties of the matter. Intermolecular forces or IMF are also known as the electrostatic forces between molecules and atoms.
Intermolecular forces exist between the molecules and affect the physical properties of a substance. The intermolecular forces of attraction are the result of the reaction between the protons or positive compounds and the electrons or negative compounds of a molecule.
Intermolecular forces examples: - Ion-dipole forces, ion-induced dipole forces, and hydrogen bonding.
The intermolecular forces depend on the following interactions:
- Dipole-Dipole Interactions
- Ion-Dipole Interactions
- Ion Induced Dipole Interactions
- Dipole Induced Dipole Interaction
- Dispersion Forces or London Forces



