Question:
For the given elementary reactions, the steady-state concentration of X is

For the given elementary reactions, the steady-state concentration of X is


Updated On: Oct 1, 2024
- \(\frac{K_1[P]^2+K_3[Q][R]}{K_2+K_4}\)
- \(\frac{\frac{1}{2}K_1[P]^2+k_3[Q][R]}{k_2+k_4}\)
- \(\frac{k_1[p]+k_3[Q][R]}{k_2+k_4}\)
- \(\frac{K_1[P]+k_3[Q][R]}{k_2+k_4}\)
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
The correct option is (A): \(\frac{K_1[P]^2+K_3[Q][R]}{K_2+K_4}\)
Was this answer helpful?
0
0
Top Questions on Chemical Kinetics
- Consider the following transformation involving first-order elementary reaction in each step at constant temperature as shown below: \[ \text{A} + \text{B} \xrightarrow{\text{Step 1}} \text{C} \xrightarrow{\text{Step 2}} \text{P}\] Some details of the above reaction are listed below:

- JEE Main - 2024
- Chemistry
- Chemical Kinetics
- Consider the following reaction, the rate expression of which is given below:
\( \text{A} + \text{B} \to \text{C} \)
\(\text{rate} = k [\text{A}]^{1/2} [\text{B}]^{1/2}\)
The reaction is initiated by taking 1M concentration of A and B each. If the rate constant (\(k\)) is \(4.6 \times 10^{-2} \, \text{s}^{-1}\), then the time taken for A to become 0.1 M is ______ sec. (nearest integer)- JEE Main - 2024
- Chemistry
- Chemical Kinetics
- For a reaction $A \xrightarrow{K_1} B \xrightarrow{K_2} C$ If the rate of formation of B is set to be zero then the concentration of B is given by:
- JEE Main - 2024
- Chemistry
- Chemical Kinetics
- Given below are two statements:
Statement I: The rate law for the reaction \[ \text{A + B} \rightarrow \text{C} \] is rate\(({r}) = k[{A}]^2[{B}]\). When the concentration of both A and B is doubled, the reaction rate is increased ``x'' times.
Statement II:

The figure is showing ``the variation in concentration against time plot'' for a ``y'' order reaction. The value of $x + y$ is _________.- JEE Main - 2024
- Chemistry
- Chemical Kinetics
- During Kinetic study of reaction 2A + B \(\mapsto \) C + D, the following results were obtained:
A[M] B[M] initial rate of
formation of DI 0.1 0.1 6.0 × 10-3 II 0.3 0.2 7.2 × 10-2 III 0.3 0.4 2.88 × 10-1 IV 0.4 0.1 2.40 × 10-2
Based on above data, overall order of the reaction is ..........- JEE Main - 2024
- Chemistry
- Chemical Kinetics
View More Questions
Questions Asked in IIT JAM CY exam
- A salt QCl of a certain metal Q is electrolyzed to its elements. 40 g of metal Q is formed at an electrode. The volume of Cl2 formed at the other electrode at 1 atm pressure and 298 K is _______ litres. (rounded off to one decimal place)
[Given: The gas constant 𝑅 = 0.082 L atm mol−1 K−1 , the molar mass of Q is 40 g mol−1 and Cl2 is assumed to be an ideal gas]- IIT JAM CY - 2024
- Electrochemistry
- Exhaustive hydrogenation of the following compound
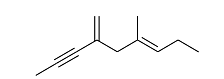
under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is _______.- IIT JAM CY - 2024
- Stereochemistry
- In the cell reaction
P+(𝑎𝑞)+Q(𝑠)→P(𝑠)+Q+(𝑎𝑞)
the EMF of the cell, 𝐸𝑐𝑒𝑙𝑙 is zero. The standard EMF of the cell, 𝐸𝑜𝑐𝑒𝑙𝑙 is
[Given:
Activities of all solids are unity.
Activity of P+(𝑎𝑞) is 2 M. Activity of Q+(𝑎𝑞) is 1 M.
𝑅 = universal gas constant; 𝑇 = temperature; 𝐹 = Faraday constant]- IIT JAM CY - 2024
- Electrochemistry
- A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
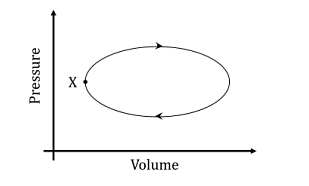
The correct statement about this process is- IIT JAM CY - 2024
- Thermodynamics
- The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:
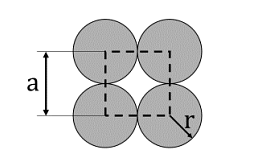
The percentage (%) area occupied by the grey circles (of radius r) inside the unit cell is _______. (rounded off to the nearest integer)- IIT JAM CY - 2024
- Solid State
View More Questions



