Considering the following reaction sequence,

the correct option(s) is(are)

the correct option(s) is(are)
The Correct Option is A, B, C
Solution and Explanation

→ P may be → H2/Pd, ethanol; Sn/HCl
→ R may be → NaNO2/HCl; HNO2
→ U may be → (i) H3PO2, (ii) KMnO4 – KOH, Δ or (i) CH3 – CH2 – OH, (ii) KMnO4 – KOH, Δ
So the correct options are A,B and C.
Top Questions on Chemical Reactions of Alcohols Phenols and Ethers
- Find out the final product C

- JEE Main - 2024
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
- Number of isomeric products formed by monochlorination Of \(2-methyl \) \(butane\) in presence of sunlight is
- JEE Main - 2024
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
- Moles of \(CH_4\) required for formation of \(22\) \(g\) of \(CO_2\) is \(m \times 10^{-2}\) The value of \(m\) is:
- JEE Main - 2024
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
- Consider the following reaction that goes from A to B in three steps as shown

below Choose the correct option:- JEE Main - 2023
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
Identify the product formed (A and E)

- JEE Main - 2023
- Chemistry
- Chemical Reactions of Alcohols Phenols and Ethers
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
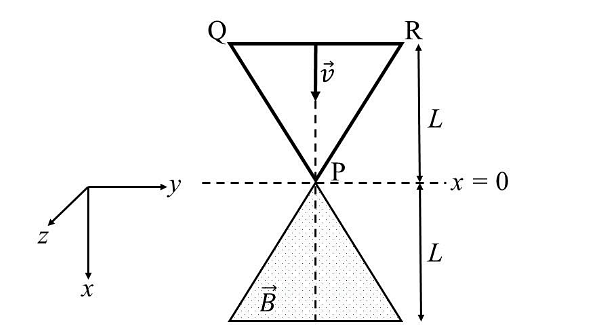
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
Concepts Used:
Chemical Properties - Alcohols, Phenols and Ethers
Alcohols, phenols, and ethers are organic compounds that have distinct chemical properties.
Alcohols are characterized by the presence of the hydroxyl (-OH) functional group, which makes them polar and capable of forming hydrogen bonds. They are typically classified as primary, secondary, or tertiary, depending on the number of alkyl groups attached to the carbon atom bearing the hydroxyl group. Alcohols undergo various chemical reactions, including oxidation, dehydration, and esterification.
Phenols are organic compounds that contain an -OH group attached to an aromatic ring. They are weaker acids than carboxylic acids but stronger acids than alcohols due to the resonance stabilization of the phenoxide ion. Phenols undergo various chemical reactions, including electrophilic substitution and oxidation.
Read More: Classification of Alcohol, Phenols and Ethers
Ethers are organic compounds that contain an oxygen atom bonded to two alkyl or aryl groups. They are characterized by their low boiling points and are often used as solvents. Ethers undergo various chemical reactions, including cleavage of the C-O bond and oxidation.
In summary, alcohols, phenols, and ethers have distinct chemical properties due to the presence of the hydroxyl or ether functional group. Understanding these properties is important for understanding their reactivity and potential applications in various fields, including chemistry, biology, and industry.







