Question:
(CH$_3 )_3$CMgCl on treatment with D$_2O$ produces
(CH$_3 )_3$CMgCl on treatment with D$_2O$ produces
Updated On: Jun 14, 2022
- $(CH_3)_3CD$
- $(CH_3)_3COD$
- $(CD)_3CD$
- $(CD)_3COD$
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
(a)
Was this answer helpful?
0
0
Top Questions on Haloalkanes and Haloarenes
- For SN2 reaction, the increasing order of the reactivity of the following alkyl halides is:
(A) CH3CH2CH2CH2Br (B) CH3CH2CH(Br)CH3 (C) (CH3)3CBr (D) (CH3)2CHCH2Br- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Match List-I with List-II:

- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Although chlorine is electron-withdrawing, it is ortho- and para-directing in electrophilic substitution because • (A) Chlorine withdraws electrons through inductive effect.
(B) Chlorine destabilizes the intermediate carbocation formed during electrophilic sub-stitution.
(C) Chlorine accepts electrons through resonance
(D) Chlorine releases electrons through resonance.
Choose the correct answer from the options given below:- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- What will be the reactivity order of following compounds towards electrophilic substitution reaction ?
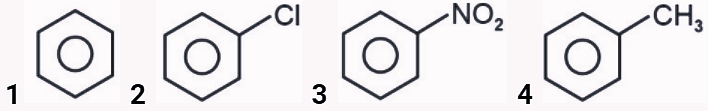
- JEE Main - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Which of the following sets of elements can be detected by Lassaigne's Test ?
- JEE Main - 2024
- Chemistry
- Haloalkanes and Haloarenes
View More Questions
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.

Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
View More Questions
Concepts Used:
Haloalkanes and Haloarenes
The hydrocarbons such as Haloalkanes and Haloarenes are the ones, in which one or more hydrogen atoms are replaced with halogen atoms. The main difference between Haloalkanes and Haloarenes is that Haloalkanes are derived from open chained hydrocarbons, also called alkanes, and Haloarenes are derived from aromatic hydrocarbons.
- Haloalkanes have hydrocarbons made up of aliphatic alkanes and one or more hydrogen atoms replaced by halogens (elements such as Chlorine, Bromine, Fluorine, Iodine, etc.) whereas, haloarenes consist of aromatic ring or rings and one or more hydrogen atoms replaced by halogens.
- In haloalkanes, the halogen atom is attached to the sp3 hybridized carbon atom of the alkyl group whereas, in haloarenes, the halogen atom is attached to the sp3 hybridized carbon atom of the alkyl group.
- Haloalkanes are saturated organic compounds where all the chemical bonds are attached to the carbon atom with single bonds and a single carbon atom is attached to the Halogen atom, whereas, the haloarenes differ from Haloalkanes by their method of preparation and properties.
- Haloalkanes are made by aliphatic alkanes by the process of free radical halogenation, whereas, haloarenes are made by direct halogenation of aromatic rings.
- Haloalkanes are odorless compounds, whereas, haloarenes have a sweet odor.
- Haloalkanes precipitate in SN2 substitution reactions, whereas, haloarenes do not precipitate in SN2 substitution reactions.
- Example of haloalkanes is CH3Cl (Methyl Chloride) and CH3CH2Br (Ethyl Bromide) and the example of haloarenes is Chlorobenzene, Bromobenzene.



