Comprehension
A trinitro compound, 1,3,5-tris-(4-nitrophenyl)benzene, on complete reaction with an excess of Sn/HCl gives a major product, which on treatment with an excess of NaNO2/HCl at 0°C provides P as the product. P, upon treatment with excess of H2O at room temperature, gives the product Q. Bromination of Q in aqueous medium furnishes the product R. The compound P upon treatment with an excess of phenol under basic conditions gives the product S. The molar mass difference between compounds Q and R is 474 g mol–1 and between compounds P and S is 172.5 g mol–1.
Question: 1
The number of heteroatoms present in one molecule of R is ______ .
[Use: Molar mass (in g mol–1): H = 1, C = 12, N = 14, O = 16, Br = 80, Cl = 35.5 Atoms other than C and H are considered as heteroatoms]
The number of heteroatoms present in one molecule of R is ______ .
[Use: Molar mass (in g mol–1): H = 1, C = 12, N = 14, O = 16, Br = 80, Cl = 35.5 Atoms other than C and H are considered as heteroatoms]
[Use: Molar mass (in g mol–1): H = 1, C = 12, N = 14, O = 16, Br = 80, Cl = 35.5 Atoms other than C and H are considered as heteroatoms]
Updated On: Mar 30, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 9
Solution and Explanation

Number of Heteroatoms in R is 9.
Was this answer helpful?
0
0
Question: 2
The total number of carbon atoms and heteroatoms present in one molecule of S is ______ .
[Use: Molar mass (in g mol–1): H = 1, C = 12, N = 14, O = 16, Br = 80, Cl = 35.5 Atoms other than C and H are considered as heteroatoms]
The total number of carbon atoms and heteroatoms present in one molecule of S is ______ .
[Use: Molar mass (in g mol–1): H = 1, C = 12, N = 14, O = 16, Br = 80, Cl = 35.5 Atoms other than C and H are considered as heteroatoms]
[Use: Molar mass (in g mol–1): H = 1, C = 12, N = 14, O = 16, Br = 80, Cl = 35.5 Atoms other than C and H are considered as heteroatoms]
Updated On: Mar 30, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 51
Solution and Explanation

Number of Carbon atoms + Number of Heteroatoms = 51
So, the answer is 51.
Was this answer helpful?
0
0
Top Questions on Organic Chemistry- Some Basic Principles and Techniques
- The compound that will undergo SN1 reaction with the fastest rate is
- NEET (UG) - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- Match List I with List II
List-I
(Conversion)List-II
(Shape/geometry)(A) 1 mol of H2O to O2 (I) 3F (B) 1 mol of MnO-4 to Mn2+ (II) 2F (C) 1.5 mol of Ca from molten CaCl2 (III) 1F (D) 1 mol of FeO to Fe2O3 (IV) 5F
Choose the correct answer from the options given below:- NEET (UG) - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- Which is following compound is easily attacked by electrophile?
- JEE Main - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- The option(s) with correct sequence of reagents for the conversion of P to Q is(are)
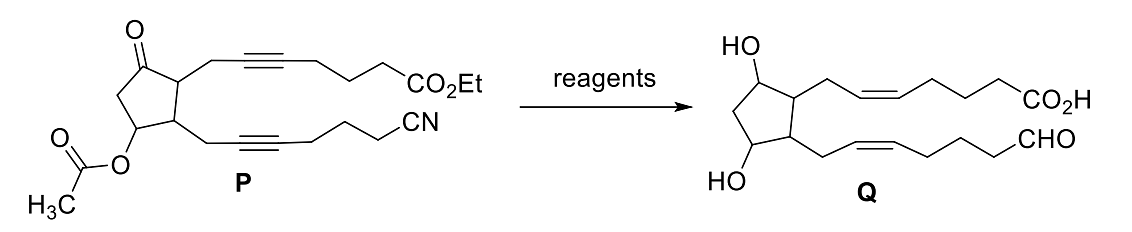
- JEE Advanced - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
- Match List I with List II
List-I
(Complex)List-II
(Type of isomerism)(A) [Co(NH3)5(NO2)]Cl2 (I) Solvate isomerism (B) [Co(NH3)5(SO4)]Br (II) Linkage isomerism (C) [Co(NH3)6] [Cr(CN)6] (III) Ionization isomerism (D) [Co(H2O)6]Cl3 (IV) Coordination isomerism
Choose the correct answer from the options given below:- NEET (UG) - 2024
- Chemistry
- Organic Chemistry- Some Basic Principles and Techniques
View More Questions
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
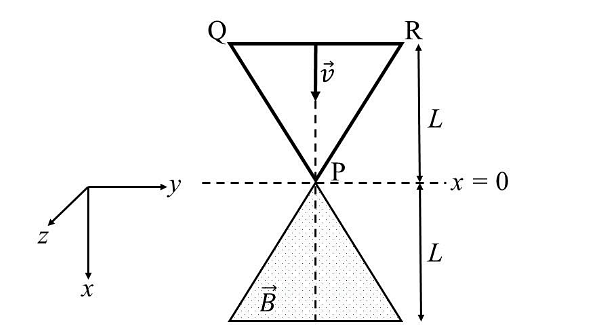
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
View More Questions



