Which of the following is NOT true about the amorphous solids ?
Which of the following is NOT true about the amorphous solids ?
- Amorphous solids can be moulded by heating.
- On heating they may become crystalline on certain temperature.
- They are anisotropic in nature
- They may become crystalline on keeping for long time.
The Correct Option is C
Solution and Explanation
The statement that is NOT true about amorphous solids is: (C) They are anisotropic in nature.
Amorphous solids do not have a well-defined long-range order like crystalline solids. They have a disordered arrangement of atoms or molecules, resulting in a lack of symmetry in their structure. Due to this lack of long-range order, amorphous solids exhibit isotropic properties, meaning their properties are the same in all directions. In contrast, crystalline solids have a well-defined periodic arrangement and can exhibit anisotropic properties, meaning their properties can vary depending on the direction.
Therefore, amorphous solids are NOT anisotropic in nature.
Top Questions on The solid state
- In a metal deficient oxide sample, MXY2O4(M and Y are metals), M is present in both +2 and +3 oxidation states and Y is in +3 oxidation state. If the fraction of M2+ ions present in M is \(\frac{1}{3}\), the value of X is______.
- JEE Advanced - 2024
- Chemistry
- The solid state
- Match List I with List II
List-I
List-II
(A) Hexagonal (I) ∝ ≠ β ≠ γ ≠ 90° (B) Orthorhombic (II) ∝ = γ = 90°, β ≠ 90° (C) Triclinic (III) ∝ = β = 90°, γ = 120° (D) Monoclinic (IV) ∝ = β = γ = 90°
Choose the correct answer from the options given below:- CUET (UG) - 2023
- Chemistry
- The solid state
- A cubic solid is made up of two elements $X$ and $Y$ Atoms of $X$ are present on every alternate corner and one at the center of cube $Y$ is at $\frac{1}{3} rd$ of the total faces. The empirical formula of the compound is
- JEE Main - 2023
- Chemistry
- The solid state
- A metal 𝑀 forms hexagonal close-packed structure. The total number of voids in 0.02 mol of it is _______ $×10^{21}$. (Nearest integer).
( Given $N_A=6.02×10^{23}$)- JEE Main - 2023
- Chemistry
- The solid state
A cubic solid is made up of two elements $X$ and $Y$ Atoms of $X$ are present on every alternate corner and one at the enter of cube $Y$ is at $\frac{1}{3} td$ of the total faces The empirical formula of the compound is
- JEE Main - 2023
- Chemistry
- The solid state
Questions Asked in KCET exam
- The current in a coil changes from 2A to 5A in 0.3s. The magnitude of emf induced in the coil is 1.0V. The value of self-inductance of the coil is
- KCET - 2023
- Electromagnetic induction
- A stretched wire of a material whose Young's modulus Y = 2 × 1011 Nm-2 has Poisson's ratio of 0.25. Its lateral strain εl = 10-3. The elastic energy density of the wire is
- KCET - 2023
- mechanical properties of solids
- A particle moves along the curve \(\frac{x^2}{16}+\frac{y^2}{4}=1\). When the rate of change of abscissa is 4 times that of its ordinate, then the quadrant in which the particle lies is
- KCET - 2023
- Conic sections
- A point object is moving at a constant speed of 1 ms-1 along the principal axis of a convex lens of focal length 10cm. The speed of the image is also 1 ms-1 , when the object is at _______ cm from the optic centre of the lens.
- KCET - 2023
- spherical lenses
- A cubical Gaussian surface has side of length a = 10 cm. Electric field lines are parallel to x-axis as shown. The magnitudes of electric fields through surfaces ABCD and EFGH are 6kNC-1 and 9kNC-1 respectively. Then the total charge enclosed by the cube is
[Take ε0 = 9 × 10-12 Fm-1]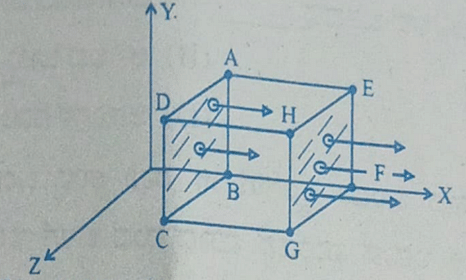
- KCET - 2023
- Gauss Law
Concepts Used:
Solid State
Solids are substances that are featured by a definite shape, volume, and high density. In the solid-state, the composed particles are arranged in several manners. Solid-state, in simple terms, means "no moving parts." Thus solid-state electronic devices are the ones inclusive of solid components that don’t change their position. Solid is a state of matter where the composed particles are arranged close to each other. The composed particles can be either atoms, molecules, or ions.
Types of Solids:
Based on the nature of the order that is present in the arrangement of their constituent particles solids can be divided into two types;
- Amorphous solids behave the same as super cool liquids due to the arrangement of constituent particles in short-range order. They are isotropic and have a broad melting point (range is about greater than 5°C).
- Crystalline solids have a fixed shape and the constituent particles are arranged in a long-range order.



