Which of the following free radicals helps in depletion of the ozone layer?
- NO
- Cl
- OH
- CH3
The Correct Option is B
Approach Solution - 1
The correct option is (B): Cl
The depletion of the ozone layer is mainly caused by the presence of free radicals containing chlorine and/or bromine atoms, which are collectively referred to as halogen radicals.
The two most important halogen radicals responsible for the depletion of the ozone layer are:
- Chlorine radical (Cl•)
- Bromine radical (Br•)
These free radicals are formed from the breakdown of chlorofluorocarbons (CFCs) and other halogenated compounds, which were widely used as refrigerants, propellants, and solvents before their harmful effects on the ozone layer were discovered.
When CFCs and other halogenated compounds are released into the atmosphere, they are carried up into the stratosphere by natural air currents. In the stratosphere, the compounds are broken down by the intense ultraviolet radiation from the sun, releasing chlorine and/or bromine atoms as free radicals.
Once released, these halogen radicals can react with ozone (O3) molecules, breaking them down into oxygen (O2) molecules and leaving behind more free radicals to continue the cycle of ozone destruction. This process is known as ozone depletion and leads to the thinning of the ozone layer.
Therefore, the free radicals that help in the depletion of the ozone layer are the halogen radicals, particularly the chlorine and bromine radicals.
Approach Solution -2

Top Questions on Environmental Chemistry
- Identify the incorrect pair from the following:
- JEE Main - 2024
- Chemistry
- Environmental Chemistry
- Which of these reactions is not a part of breakdown of ozone in stratosphere?
- JEE Main - 2023
- Chemistry
- Environmental Chemistry
- Formation of photochemical smog involves the following reaction in which $A , B$ and $C$ are respectively i $NO _2 \xrightarrow{ h\nu } A + B$ ii $B + O _2 \rightarrow C$ iii $A + C \rightarrow NO _2+ O _2$ Choose the correct answer from the options given below:
- JEE Main - 2023
- Chemistry
- Environmental Chemistry
- In which of the following cities photochemical smog is minimum?
- JEE Main - 2023
- Chemistry
- Environmental Chemistry
- Consider the following:
- D.D.T.
- Aldrin
- Sodium arsenite
- Sodium chlorate
- JEE Main - 2023
- Chemistry
- Environmental Chemistry
Questions Asked in JEE Main exam
- Let \[\vec{a} = \hat{i} + \hat{j} + \hat{k}, \quad \vec{b} = -\hat{i} - 8\hat{j} + 2\hat{k}, \quad \text{and} \quad \vec{c} = 4\hat{i} + c_2\hat{j} + c_3\hat{k} \]be three vectors such that \[\vec{b} \times \vec{a} = \vec{c} \times \vec{a}.\]If the angle between the vector $\vec{c}$ and the vector $3\hat{i} + 4\hat{j} + \hat{k}$ is $\theta$, then the greatest integer less than or equal to $\tan^2 \theta$ is:
- JEE Main - 2024
- Vector Algebra
- 10 mL of gaseous hydrocarbon on combustion gives 40 mL of CO\(_2\)(g) and 50 mL of water vapour. The total number of carbon and hydrogen atoms in the hydrocarbon is ______ .
- JEE Main - 2024
- Hydrocarbons
- If each term of a geometric progression \( a_1, a_2, a_3, \dots \) with \( a_1 = \frac{1}{8} \) and \( a_2 \neq a_1 \), is the arithmetic mean of the next two terms and \( S_n = a_1 + a_2 + \dots + a_n \), then \( S_{20} - S_{18} \) is equal to
- JEE Main - 2024
- Arithmetic Mean
A body of mass 1000 kg is moving horizontally with a velocity of 6 m/s. If 200 kg extra mass is added, the final velocity (in m/s) is:
- JEE Main - 2024
- speed and velocity
- $\textbf{Choose the correct statements about the hydrides of group 15 elements.}$
A. The stability of the hydrides decreases in the order \(\text{NH}_3 > \text{PH}_3 > \text{AsH}_3 > \text{SbH}_3 > \text{BiH}_3\)
B. The reducing ability of the hydrides increases in the order \(\text{NH}_3 < \text{PH}_3 < \text{AsH}_3 < \text{SbH}_3 < \text{BiH}_3\)
C. Among the hydrides, \(\text{NH}_3\) is a strong reducing agent while \(\text{BiH}_3\) is a mild reducing agent.
D. The basicity of the hydrides increases in the order \(\text{NH}_3 < \text{PH}_3 < \text{AsH}_3 < \text{SbH}_3 < \text{BiH}_3\)
Choose the most appropriate from the option given below:- JEE Main - 2024
- p -Block Elements
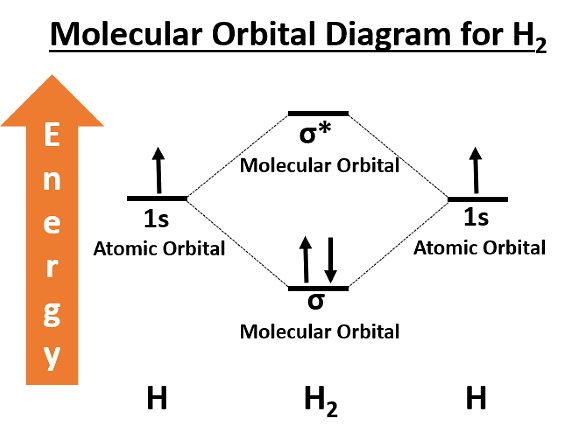
Concepts Used:
Molecular Orbital Theory
The Molecular Orbital Theory is a more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO).
Molecular Orbital theory is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Due to this arrangement in MOT Theory, electrons associated with different nuclei can be found in different atomic orbitals. In molecular orbital theory, the electrons present in a molecule are not assigned to individual chemical bonds between the atoms. Rather, they are treated as moving under the influence of the atomic nuclei in the entire molecule.