Question:
Which of the following does not have S-S bond?
Which of the following does not have S-S bond?
Updated On: Mar 17, 2023
- $ {{S}_{2}}O_{3}^{2-} $
- $ {{S}_{2}}O_{6}^{2-} $
\({{S}_{2}}O_{5}^{2-}\)
- $ {{S}_{2}}O_{7}^{2-} $
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
$ {{S}_{2}}O_{7}^{2-} $ (pyrosulphate ion) has no sulphur-sulphur bond. Its structure is as follows:
Was this answer helpful?
0
0
Top Questions on Group 16 Elements
- Choose the correct statements from the following:
A. All group 16 elements form oxides of general formula \( EO_2 \) and \( EO_3 \) where \( E = S, Se, Te, \) and Po. Both the types of oxides are acidic in nature.
B. TeO\(_2\) is an oxidising agent while SO\(_2\) is reducing in nature.
C. The reducing property decreases from H\(_2\)S to H\(_2\)Te down the group.
D. The ozone molecule contains five lone pairs of electrons.
Choose the correct answer from the options given below:- JEE Main - 2024
- Chemistry
- Group 16 Elements
- From the given list, the number of compounds with +4 oxidation state of Sulphur_____.
\(SO_3, H_2SO_3, SOCl_2, SF_4, BaSO_4, H_2S_2O_7\).- JEE Main - 2024
- Chemistry
- Group 16 Elements
- Given below are two statements :
Statement I: The boiling point of hydrides of Group 16 elements follow the order
H2O > H2Te > H2Se > H2S.
Statement II: On the basis of molecular mass, H2O is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in H2O, it has higher boiling point.
In the light of the above statements, choose the correct answer from the options given below :- NEET (UG) - 2024
- Chemistry
- Group 16 Elements
- Among Group 16 elements, which one does NOT show -2 oxidation state?
- NEET (UG) - 2024
- Chemistry
- Group 16 Elements
- Why does oxygen show anomalous behavior ?
- JEE Main - 2024
- Chemistry
- Group 16 Elements
View More Questions
Questions Asked in JIPMER exam
- Binomial nomenclature was first introduced by
- JIPMER - 2015
- KCET - 2022
- Diversity In The Living World
- A convex lens $'A'$ of focal length $20\, cm$ and a concave lens $'B'$ of focal length $5\, cm$ are kept along the same axis with a distance $'d'$ between them. If a parallel beam of light falling on $'A'$ leaves $'B'$ as a parallel beam, then the distance $'d'$ in cm will be :
- JIPMER - 2021
- Spherical Mirrors
- A bacterial flagellum is composed of
- JIPMER - 2021
- Prokaryotic Cells
- $2,4-DNP $ test can be used to identify :
- JIPMER - 2021
- Chemical Reactions
- How many moles of acidified $K_2Cr_2O_7$ is required to liberate $6$ moles of $I_2$ from an aqueous solution of $I^-$ ?
- JIPMER - 2020
- Mole concept and Molar Masses
View More Questions
Concepts Used:
Group 16 Elements
The group 16 elements (oxygen group elements) of the periodic classification are also known as chalcogens because most of the copper ores have copper in the form of oxides and sulphides. The word chalcogen means “ore formation” which is derived from the Greek word “Chalcos” (Ore) and “gen” (formation).
There are 5 elements that come under Group 16 of the Modern Periodic Table namely:
- Oxygen (O)
- Sulphur (S)
- Selenium (Se)
- Tellurium (Te)
- Polonium (PO)
Electronic Configuration:
The general electronic configuration of the chalcogens can be written as ‘ns2np4’, where ‘n’ denotes the value of the principal quantum number corresponding to the valence shell of the element.
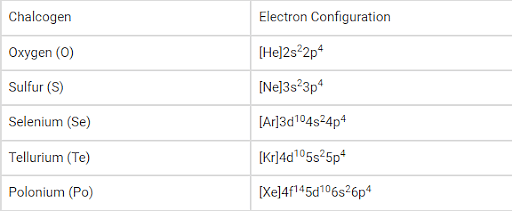
The electron configuration of the synthetic element livermorium (believed to be a chalcogen) is predicted to be [Rn]5f146d107s27p4.
Metallic Nature of the Group 16 Elements:
- Oxygen and sulfur are classified as non-metals.
- Selenium and tellurium are classified as metalloids.
- Under standard conditions, polonium exhibits metallic characteristics. However, it is important to note that polonium is a radioactive element.



