Question:
Vessel-1 contains w2 g of a non-volatile solute X dissolved in w1 g of water. Vessel-2 contains w2 g of another non-volatile solute Y dissolved in w1 g of water. Both the vessels are at the same temperature and pressure. The molar mass of X is 80% of that of Y. The van’t Hoff factor for X is 1.2 times of that of Y for their respective concentrations.
The elevation of boiling point for solution in Vessel-1 is _____ % of the solution in Vessel-2.
Vessel-1 contains w2 g of a non-volatile solute X dissolved in w1 g of water. Vessel-2 contains w2 g of another non-volatile solute Y dissolved in w1 g of water. Both the vessels are at the same temperature and pressure. The molar mass of X is 80% of that of Y. The van’t Hoff factor for X is 1.2 times of that of Y for their respective concentrations.
The elevation of boiling point for solution in Vessel-1 is _____ % of the solution in Vessel-2.
The elevation of boiling point for solution in Vessel-1 is _____ % of the solution in Vessel-2.
Updated On: Jun 20, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 150
Solution and Explanation
The correct answer is: 150
Was this answer helpful?
0
0
Top Questions on Solutions
- Which term of molar conductivity is used when the concentration of electrolyte approaches zero?
- Molal elevation constant is also known as:
- Which of the following ions will be coloured in the aqueous solution?
(A) Ti3+
(B) Nb3+
(C) Cu+
(D) Y3+
Choose the correct answer from the options given below: - Which metal is the most powerful reducing agent in aqueous solution?
- A perfectly ideal solution is rare but some solutions behave nearly ideal. Which of the following does not fall in this category?
View More Questions
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
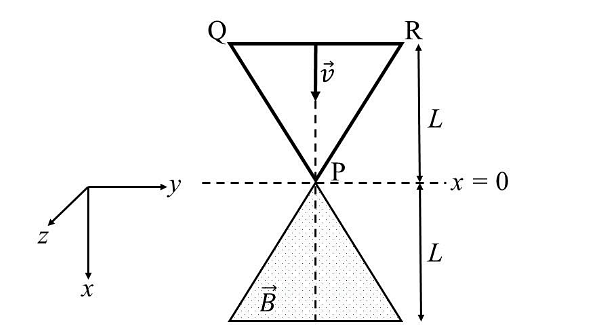
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
View More Questions



